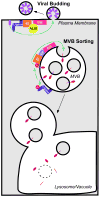
Membrane protein targeting to the MVB/lysosome
- PMID: 19243135
- PMCID: PMC3911787
- DOI: 10.1021/cr800473s
Membrane protein targeting to the MVB/lysosome
Figures


Similar articles
-
Endosomal transport via ubiquitination.Trends Cell Biol. 2011 Nov;21(11):647-55. doi: 10.1016/j.tcb.2011.08.007. Epub 2011 Sep 28. Trends Cell Biol. 2011. PMID: 21955996 Free PMC article. Review.
-
RNF167 targets Arl8B for degradation to regulate lysosome positioning and endocytic trafficking.FEBS J. 2016 Dec;283(24):4583-4599. doi: 10.1111/febs.13947. Epub 2016 Nov 16. FEBS J. 2016. PMID: 27808481
-
Biogenesis and function of multivesicular bodies.Annu Rev Cell Dev Biol. 2007;23:519-47. doi: 10.1146/annurev.cellbio.23.090506.123319. Annu Rev Cell Dev Biol. 2007. PMID: 17506697 Free PMC article. Review.
-
LAPTM4α is targeted from the Golgi to late endosomes/lysosomes in a manner dependent on the E3 ubiquitin ligase Nedd4-1 and ESCRT proteins.Biochem Biophys Res Commun. 2021 Jun 4;556:9-15. doi: 10.1016/j.bbrc.2021.03.151. Epub 2021 Apr 6. Biochem Biophys Res Commun. 2021. PMID: 33836347
-
Ubiquitin and endocytic protein sorting.Essays Biochem. 2005;41:81-98. doi: 10.1042/EB0410081. Essays Biochem. 2005. PMID: 16250899 Review.
Cited by
-
The Endosome-associated Deubiquitinating Enzyme USP8 Regulates BACE1 Enzyme Ubiquitination and Degradation.J Biol Chem. 2016 Jul 22;291(30):15753-66. doi: 10.1074/jbc.M116.718023. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302062 Free PMC article.
-
Coordination of substrate binding and ATP hydrolysis in Vps4-mediated ESCRT-III disassembly.Mol Biol Cell. 2010 Oct 1;21(19):3396-408. doi: 10.1091/mbc.E10-06-0512. Epub 2010 Aug 11. Mol Biol Cell. 2010. PMID: 20702581 Free PMC article.
-
ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1015-27. doi: 10.1152/ajpcell.00120.2010. Epub 2010 Aug 18. Am J Physiol Cell Physiol. 2010. PMID: 20720181 Free PMC article.
-
Multivesicular bodies in neurons: distribution, protein content, and trafficking functions.Prog Neurobiol. 2011 Mar;93(3):313-40. doi: 10.1016/j.pneurobio.2011.01.003. Epub 2011 Jan 7. Prog Neurobiol. 2011. PMID: 21216273 Free PMC article. Review.
-
Cancer Extracellular Vesicles: Next-Generation Diagnostic and Drug Delivery Nanotools.Cancers (Basel). 2020 Oct 28;12(11):3165. doi: 10.3390/cancers12113165. Cancers (Basel). 2020. PMID: 33126572 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

