Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects
- PMID: 19240021
- PMCID: PMC2670154
- DOI: 10.1074/jbc.M809233200
Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects
Abstract
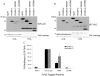
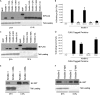
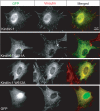
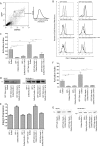
Integrin activation, the rapid conversion of integrin adhesion receptors from low to high affinity, occurs in response to intracellular signals that act on the short cytoplasmic tails of integrin beta subunits. Talin binding to integrin beta tails provides one key activation signal, but additional factors are likely to cooperate with talin to regulate integrin activation. The integrin beta tail-binding proteins kindlin-2 and kindlin-3 were recently identified as integrin co-activators. Here we report an analysis of kindlin-1 and kindlin-2 interactions with beta1 and beta3 integrin tails and describe the effect of kindlin expression on integrin activation. We demonstrate a direct interaction of kindlin-1 and -2 with recombinant integrin beta tails in pulldown binding assays. Our mutational analysis shows that the second conserved NXXY motif (Tyr(795)), a preceding threonine-containing region (Thr(788) and Thr(789)) of the integrin beta1A tail, and a conserved tryptophan in the F3 subdomain of the kindlin FERM domain (kindlin-1 Trp(612) and kindlin-2 Trp(615)) are required for direct kindlin-integrin interactions. Similar interactions were observed for integrin beta3 tails. Using fluorescence-activated cell sorting we further show that transient expression of kindlin-1 or -2 in Chinese hamster ovary cells inhibits the activation of endogenous alpha5beta1 or stably expressed alphaIIbbeta3 integrins. This inhibition is not dependent on direct kindlin-integrin interactions because mutant kindlins exhibiting impaired integrin binding activity effectively inhibit integrin activation. Consistent with previous reports, we find that when co-expressed with the talin head, kindlin-1 or -2 can activate alphaIIbbeta3. This effect is dependent on an intact integrin-binding site in kindlin. Notably however, even when co-expressed with activating levels of talin head, neither kindlin-1 or -2 can cooperate with talin to activate beta1 integrins; instead they strongly inhibit talin-mediated activation. We suggest that kindlins are adaptor proteins that regulate integrin activation, that kindlin expression levels determine their effects, and that kindlins may exert integrin-specific effects.
Figures



Similar articles
-
A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation.J Biol Chem. 2012 Mar 2;287(10):6979-90. doi: 10.1074/jbc.M111.330845. Epub 2012 Jan 10. J Biol Chem. 2012. PMID: 22235127 Free PMC article.
-
Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β cytoplasmic tails.J Biol Chem. 2012 Jul 13;287(29):24585-94. doi: 10.1074/jbc.M111.336743. Epub 2012 May 30. J Biol Chem. 2012. PMID: 22648415 Free PMC article.
-
Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3.PLoS One. 2012;7(3):e34056. doi: 10.1371/journal.pone.0034056. Epub 2012 Mar 23. PLoS One. 2012. PMID: 22457811 Free PMC article.
-
Kindlin: helper, co-activator, or booster of talin in integrin activation?Curr Opin Hematol. 2011 Sep;18(5):356-60. doi: 10.1097/MOH.0b013e3283497f09. Curr Opin Hematol. 2011. PMID: 21730832 Review.
-
Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart.Pediatr Cardiol. 2019 Oct;40(7):1401-1409. doi: 10.1007/s00246-019-02167-3. Epub 2019 Jul 31. Pediatr Cardiol. 2019. PMID: 31367953 Free PMC article. Review.
Cited by
-
Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions.Nat Commun. 2021 May 25;12(1):3104. doi: 10.1038/s41467-021-23372-w. Nat Commun. 2021. PMID: 34035280 Free PMC article.
-
Talin and signaling through integrins.Methods Mol Biol. 2012;757:325-47. doi: 10.1007/978-1-61779-166-6_20. Methods Mol Biol. 2012. PMID: 21909921 Free PMC article.
-
Functional Effect of the Mutations Similar to the Cleavage during Platelet Activation at Integrin β3 Cytoplasmic Tail when Expressed in Mouse Platelets.PLoS One. 2016 Nov 16;11(11):e0166136. doi: 10.1371/journal.pone.0166136. eCollection 2016. PLoS One. 2016. PMID: 27851790 Free PMC article.
-
Protein 4.1G Regulates Cell Adhesion, Spreading, and Migration of Mouse Embryonic Fibroblasts through the β1 Integrin Pathway.J Biol Chem. 2016 Jan 29;291(5):2170-80. doi: 10.1074/jbc.M115.658591. Epub 2015 Dec 7. J Biol Chem. 2016. PMID: 26644476 Free PMC article.
-
Emergence and subsequent functional specialization of kindlins during evolution of cell adhesiveness.Mol Biol Cell. 2015 Feb 15;26(4):786-96. doi: 10.1091/mbc.E14-08-1294. Epub 2014 Dec 24. Mol Biol Cell. 2015. PMID: 25540429 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

