Coordinated implementation of chikungunya virus reverse transcription-PCR
- PMID: 19239767
- PMCID: PMC2681123
- DOI: 10.3201/eid1503.081104
Coordinated implementation of chikungunya virus reverse transcription-PCR
Erratum in
- Emerg Infect Dis. 2009 Aug;15(8):1334. Mantke, Oliver D [corrected to Donoso Mantke, Oliver]
Abstract
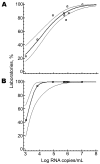
A preformulated chikungunya virus real-time reverse transcription-PCR, quality-confirmed oligonucleotides, and noninfectious virus controls were distributed by the European Network for the Diagnosis of Imported Viral Diseases. An international proficiency study with 31 participants demonstrated that ad hoc implementation of molecular diagnostics was feasible and successful.
Figures
Similar articles
-
Performance of the RealStar Chikungunya virus real-time reverse transcription-PCR kit.J Clin Microbiol. 2009 Sep;47(9):3014-6. doi: 10.1128/JCM.01024-09. Epub 2009 Jul 22. J Clin Microbiol. 2009. PMID: 19625474 Free PMC article.
-
Rapid detection and quantification of Chikungunya virus by a one-step reverse transcription polymerase chain reaction real-time assay.Am J Trop Med Hyg. 2007 Sep;77(3):521-4. Am J Trop Med Hyg. 2007. PMID: 17827371
-
Utility of multiplex reverse transcriptase-polymerase chain reaction for diagnosis and serotypic characterization of dengue and chikungunya viruses in clinical samples.Diagn Microbiol Infect Dis. 2011 Oct;71(2):118-25. doi: 10.1016/j.diagmicrobio.2011.06.020. Epub 2011 Aug 23. Diagn Microbiol Infect Dis. 2011. PMID: 21865001
-
Development of a quantitative competitive reverse transcription polymerase chain reaction (QC-RT-PCR) for detection and quantitation of Chikungunya virus.Mol Biotechnol. 2010 May;45(1):49-55. doi: 10.1007/s12033-009-9238-9. Mol Biotechnol. 2010. PMID: 20054667
-
[Chikungunya fever].Bing Du Xue Bao. 2011 Jul;27(4):372-7. Bing Du Xue Bao. 2011. PMID: 21874908 Review. Chinese. No abstract available.
Cited by
-
A high-throughput screening assay to identify inhibitory antibodies targeting alphavirus release.Virol J. 2022 Oct 29;19(1):170. doi: 10.1186/s12985-022-01906-y. Virol J. 2022. PMID: 36309730 Free PMC article.
-
Detection of respiratory viruses using a multiplex real-time PCR assay in Germany, 2009/10.Arch Virol. 2014 Apr;159(4):669-76. doi: 10.1007/s00705-013-1876-3. Epub 2013 Oct 15. Arch Virol. 2014. PMID: 24126621 Free PMC article.
-
Analysis of a Routinely Used Commercial Anti-Chikungunya IgM ELISA Reveals Cross-Reactivities with Dengue in Brazil: A New Challenge for Differential Diagnosis?Diagnostics (Basel). 2021 Apr 30;11(5):819. doi: 10.3390/diagnostics11050819. Diagnostics (Basel). 2021. PMID: 33946597 Free PMC article.
-
Evaluation of Chikungunya diagnostic assays: differences in sensitivity of serology assays in two independent outbreaks.PLoS Negl Trop Dis. 2010 Jul 20;4(7):e753. doi: 10.1371/journal.pntd.0000753. PLoS Negl Trop Dis. 2010. PMID: 20651930 Free PMC article.
-
A taRNA vaccine candidate induces a specific immune response that protects mice against Chikungunya virus infections.Mol Ther Nucleic Acids. 2022 May 2;28:743-754. doi: 10.1016/j.omtn.2022.04.036. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35664702 Free PMC article.
References
-
- Krastinova E, Quatresous I, Tarantola A. Imported cases of chikungunya in metropolitan France: update to June 2006. Euro Surveill. 2006;11:E060824.1. - PubMed
-
- Sang RC, Ahmed O, Faye O, Kelly CL, Yahaya AA, Mmadi J, et al. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am J Trop Med Hyg. 2008;78:77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
