Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background
- PMID: 19237661
- PMCID: PMC2656413
- DOI: 10.1161/CIRCULATIONAHA.108.798983
Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background
Abstract
Background: Cardiac myosin binding protein-C (cMyBP-C) phosphorylation modulates cardiac contractility. When expressed in cMyBP-C-null (cMyBP-C((t/t))) hearts, a cMyBP-C phosphomimetic (cMyBP-C(AllP+)) rescued cardiac dysfunction and protected the hearts from ischemia/reperfusion injury. However, cMyBP-C function may be dependent on the myosin isoform type. Because these replacements were performed in the mouse heart, which contains predominantly alpha-myosin heavy chain (alpha-MyHC), the applicability of the data to humans, whose cardiomyocytes contain predominantly beta-MyHC, is unclear. We determined the effect(s) of cMyBP-C phosphorylation in a beta-MyHC transgenic mouse heart in which >80% of the alpha-MyHC was replaced by beta-MyHC, which is the predominant myosin isoform in human cardiac muscle.
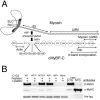
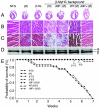
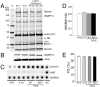
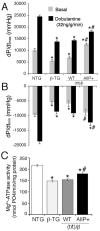
Methods and results: To determine the effects of cMyBP-C phosphorylation in a beta-MyHC background, transgenic mice expressing normal cMyBP-C (cMyBP-C(WT)), nonphosphorylatable cMyBP-C (cMyBP-C(AllP)(-)), or cMyBP-C(AllP+) were bred into the beta-MyHC background (beta). These mice were then crossed into the cMyBP-C((t/t)) background to ensure the absence of endogenous cMyBP-C. cMyBP-C((t/t)/beta) and cMyBP-C(AllP)(-)(:(t/t)/beta) mice died prematurely because of heart failure, confirming that cMyBP-C phosphorylation is essential in the beta-MyHC background. cMyBP-C(AllP+:(t/t)/beta) and cMyBP-C(WT:(t/t)/beta) hearts showed no morbidity and mortality, and cMyBP-C(AllP+:(t/t)/beta) hearts were significantly cardioprotected from ischemia/reperfusion injury.
Conclusions: cMyBP-C phosphorylation is necessary for basal myocardial function in the beta-MyHC background and can preserve function after ischemia/reperfusion injury. Our studies justify exploration of cMyBP-C phosphorylation as a therapeutic target in the human heart.
Figures

Similar articles
-
Cardiac myosin binding protein C phosphorylation is cardioprotective.Proc Natl Acad Sci U S A. 2006 Nov 7;103(45):16918-23. doi: 10.1073/pnas.0607069103. Epub 2006 Oct 30. Proc Natl Acad Sci U S A. 2006. PMID: 17075052 Free PMC article.
-
Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation.Circulation. 2007 Nov 20;116(21):2399-408. doi: 10.1161/CIRCULATIONAHA.107.706523. Epub 2007 Nov 5. Circulation. 2007. PMID: 17984378
-
Cardiac myosin-binding protein-C phosphorylation and cardiac function.Circ Res. 2005 Nov 25;97(11):1156-63. doi: 10.1161/01.RES.0000190605.79013.4d. Epub 2005 Oct 13. Circ Res. 2005. PMID: 16224063 Free PMC article.
-
Phosphorylation and function of cardiac myosin binding protein-C in health and disease.J Mol Cell Cardiol. 2010 May;48(5):866-75. doi: 10.1016/j.yjmcc.2009.11.014. Epub 2009 Dec 3. J Mol Cell Cardiol. 2010. PMID: 19962384 Free PMC article. Review.
-
Cardiac myosin binding protein-C as a central target of cardiac sarcomere signaling: a special mini review series.Pflugers Arch. 2014 Feb;466(2):195-200. doi: 10.1007/s00424-013-1396-8. Epub 2013 Nov 7. Pflugers Arch. 2014. PMID: 24196566 Free PMC article. Review.
Cited by
-
β-adrenergic effects on cardiac myofilaments and contraction in an integrated rabbit ventricular myocyte model.J Mol Cell Cardiol. 2015 Apr;81:162-75. doi: 10.1016/j.yjmcc.2015.02.014. Epub 2015 Feb 25. J Mol Cell Cardiol. 2015. PMID: 25724724 Free PMC article.
-
Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction.Am J Cardiovasc Dis. 2013 Jun 10;3(2):60-70. Print 2013. Am J Cardiovasc Dis. 2013. PMID: 23785583 Free PMC article.
-
Myofibroblast-Specific TGFβ Receptor II Signaling in the Fibrotic Response to Cardiac Myosin Binding Protein C-Induced Cardiomyopathy.Circ Res. 2018 Dec 7;123(12):1285-1297. doi: 10.1161/CIRCRESAHA.118.313089. Circ Res. 2018. PMID: 30566042 Free PMC article.
-
RLC phosphorylation amplifies Ca2+ sensitivity of force in myocardium from cMyBP-C knockout mice.J Gen Physiol. 2023 Apr 3;155(4):e202213250. doi: 10.1085/jgp.202213250. Epub 2023 Jan 30. J Gen Physiol. 2023. PMID: 36715675 Free PMC article.
-
Signaling and myosin-binding protein C.J Biol Chem. 2011 Mar 25;286(12):9913-9. doi: 10.1074/jbc.R110.171801. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257752 Free PMC article. Review.
References
-
- Mohamed AS, Dignam JD, Schlender KK. Cardiac myosin-binding protein C (MyBP-C): identification of protein kinase A and protein kinase C phosphorylation sites. Arch Biochem Biophys. 1998;358:313–319. - PubMed
-
- Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997;336:775–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 HL074728-050004/HL/NHLBI NIH HHS/United States
- P01 HL059408/HL/NHLBI NIH HHS/United States
- P30 HD028827-109005/HD/NICHD NIH HHS/United States
- P01 HL069779-06A1/HL/NHLBI NIH HHS/United States
- P50HL074728/HL/NHLBI NIH HHS/United States
- P01HL69799/HL/NHLBI NIH HHS/United States
- P50 HL077101-040005/HL/NHLBI NIH HHS/United States
- P01 HL069779-06A17080/HL/NHLBI NIH HHS/United States
- P50HL077101/HL/NHLBI NIH HHS/United States
- P01 HL069779-06A10001/HL/NHLBI NIH HHS/United States
- P01 HL069779-040001/HL/NHLBI NIH HHS/United States
- P50 HL077101-020005/HL/NHLBI NIH HHS/United States
- P50 HL077101-010005/HL/NHLBI NIH HHS/United States
- P01 HL069779/HL/NHLBI NIH HHS/United States
- R01HL087862/HL/NHLBI NIH HHS/United States
- P50 HL077101-030005/HL/NHLBI NIH HHS/United States
- P50 HL074728/HL/NHLBI NIH HHS/United States
- R01 HL087862/HL/NHLBI NIH HHS/United States
- P01HL059408/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

