Neuron-derived FGF9 is essential for scaffold formation of Bergmann radial fibers and migration of granule neurons in the cerebellum
- PMID: 19232523
- PMCID: PMC3262992
- DOI: 10.1016/j.ydbio.2009.02.011
Neuron-derived FGF9 is essential for scaffold formation of Bergmann radial fibers and migration of granule neurons in the cerebellum
Abstract
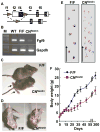
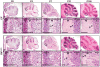
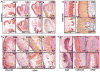
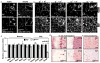
Although fibroblast growth factor 9 (FGF9) is widely expressed in the central nervous system (CNS), the function of FGF9 in neural development remains undefined. To address this question, we deleted the Fgf9 gene specifically in the neural tube and demonstrated that FGF9 plays a key role in the postnatal migration of cerebellar granule neurons. Fgf9-null mice showed severe ataxia associated with disrupted Bergmann fiber scaffold formation, impaired granule neuron migration, and upset Purkinje cell maturation. Ex vivo cultured wildtype or Fgf9-null glia displayed a stellate morphology. Coculture with wildtype neurons, but not Fgf9-deficient neurons, or treating with FGF1 or FGF9 induced the cells to adopt a radial glial morphology. In situ hybridization showed that Fgf9 was expressed in neurons and immunostaining revealed that FGF9 was broadly distributed in both neurons and Bergmann glial radial fibers. Genetic analyses revealed that the FGF9 activities in cerebellar development are primarily transduced by FGF receptors 1 and 2. Furthermore, inhibition of the MAP kinase pathway, but not the PI3K/AKT pathway, abrogated the FGF activity to induce glial morphological changes, suggesting that the activity is mediated by the MAP kinase pathway. This work demonstrates that granule neurons secrete FGF9 to control formation of the Bergmann fiber scaffold, which in turn, guides their own inward migration and maturation of Purkinje cells.
Figures



Similar articles
-
FGF9 knockout in GABAergic neurons induces apoptosis and inflammation via the Fas/caspase-3 pathway in the cerebellum of mice.Brain Res Bull. 2020 Jan;154:91-101. doi: 10.1016/j.brainresbull.2019.10.012. Epub 2019 Nov 11. Brain Res Bull. 2020. PMID: 31726090
-
Close homologue of adhesion molecule L1 promotes survival of Purkinje and granule cells and granule cell migration during murine cerebellar development.J Comp Neurol. 2009 Apr 10;513(5):496-510. doi: 10.1002/cne.21981. J Comp Neurol. 2009. PMID: 19226508
-
Transcriptional Regulator ZEB2 Is Essential for Bergmann Glia Development.J Neurosci. 2018 Feb 7;38(6):1575-1587. doi: 10.1523/JNEUROSCI.2674-17.2018. Epub 2018 Jan 11. J Neurosci. 2018. PMID: 29326173 Free PMC article.
-
Bergmann glia function in granule cell migration during cerebellum development.Mol Neurobiol. 2013 Apr;47(2):833-44. doi: 10.1007/s12035-013-8405-y. Epub 2013 Jan 19. Mol Neurobiol. 2013. PMID: 23329344 Review.
-
Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells.Anat Sci Int. 2002 Jun;77(2):94-108. doi: 10.1046/j.0022-7722.2002.00021.x. Anat Sci Int. 2002. PMID: 12418089 Review.
Cited by
-
ERBB3-mediated regulation of Bergmann glia proliferation in cerebellar lamination.Development. 2015 Feb 1;142(3):522-32. doi: 10.1242/dev.115931. Epub 2015 Jan 6. Development. 2015. PMID: 25564653 Free PMC article.
-
FGF9 is required for Purkinje cell development and function in the cerebellum.iScience. 2024 Jan 26;27(2):109039. doi: 10.1016/j.isci.2024.109039. eCollection 2024 Feb 16. iScience. 2024. PMID: 38352230 Free PMC article.
-
Sprouty genes prevent excessive FGF signalling in multiple cell types throughout development of the cerebellum.Development. 2011 Jul;138(14):2957-68. doi: 10.1242/dev.063784. Development. 2011. PMID: 21693512 Free PMC article.
-
Radial glia, the keystone of the development of the hippocampal dentate gyrus.Mol Neurobiol. 2015 Feb;51(1):131-41. doi: 10.1007/s12035-014-8692-y. Epub 2014 Apr 10. Mol Neurobiol. 2015. PMID: 24719081 Review.
-
Signaling in cell differentiation and morphogenesis.Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a008151. doi: 10.1101/cshperspect.a008151. Cold Spring Harb Perspect Biol. 2012. PMID: 22570373 Free PMC article. Review.
References
-
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–72. - PubMed
-
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–809. - PubMed
-
- Blak AA, Naserke T, Saarimaki-Vire J, Peltopuro P, Giraldo-Velasquez M, Vogt Weisenhorn DM, Prakash N, Sendtner M, Partanen J, Wurst W. Fgfr2 and Fgfr3 are not required for patterning and maintenance of the midbrain and anterior hindbrain. Dev Biol. 2007;303:231–43. - PubMed
-
- Chanas-Sacre G, Rogister B, Moonen G, Leprince P. Radial glia phenotype: origin, regulation, and transdifferentiation. J Neurosci Res. 2000;61:357–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA096824-07/CA/NCI NIH HHS/United States
- R01 CA113750/CA/NCI NIH HHS/United States
- R01 CA096824-02/CA/NCI NIH HHS/United States
- R56 CA096824/CA/NCI NIH HHS/United States
- R01 CA096824-09/CA/NCI NIH HHS/United States
- R01 CA096824-01A1/CA/NCI NIH HHS/United States
- R56 CA096824-06A1/CA/NCI NIH HHS/United States
- R01 CA096824-03/CA/NCI NIH HHS/United States
- R01 CA096824-05/CA/NCI NIH HHS/United States
- R01 CA096824-06A2/CA/NCI NIH HHS/United States
- R01 CA096824-08/CA/NCI NIH HHS/United States
- R01 CA096824-04/CA/NCI NIH HHS/United States
- R01 CA096824/CA/NCI NIH HHS/United States
- CA96824/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

