Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein
- PMID: 19228951
- PMCID: PMC2803056
- DOI: 10.1523/JNEUROSCI.5351-08.2009
Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein
Abstract
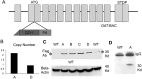
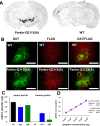
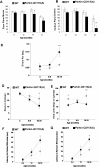
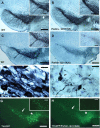
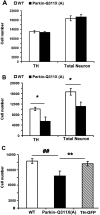
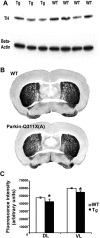
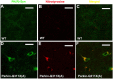
Recessive mutations in parkin are the most common cause of familial early-onset Parkinson's disease (PD). Recent studies suggest that certain parkin mutants may exert dominant toxic effects to cultured cells and such dominant toxicity can lead to progressive dopaminergic (DA) neuron degeneration in Drosophila. To explore whether mutant parkin could exert similar pathogenic effects to mammalian DA neurons in vivo, we developed a BAC (bacterial artificial chromosome) transgenic mouse model expressing a C-terminal truncated human mutant parkin (Parkin-Q311X) in DA neurons driven by a dopamine transporter promoter. Parkin-Q311X mice exhibit multiple late-onset and progressive hypokinetic motor deficits. Stereological analyses reveal that the mutant mice develop age-dependent DA neuron degeneration in substantia nigra accompanied by a significant loss of DA neuron terminals in the striatum. Neurochemical analyses reveal a significant reduction of the striatal dopamine level in mutant mice, which is significantly correlated with their hypokinetic motor deficits. Finally, mutant Parkin-Q311X mice, but not wild-type controls, exhibit age-dependent accumulation of proteinase K-resistant endogenous alpha-synuclein in substantia nigra and colocalized with 3-nitrotyrosine, a marker for oxidative protein damage. Hence, our study provides the first mammalian genetic evidence that dominant toxicity of a parkin mutant is sufficient to elicit age-dependent hypokinetic motor deficits and DA neuron loss in vivo, and uncovers a causal relationship between dominant parkin toxicity and progressive alpha-synuclein accumulation in DA neurons. Our study underscores the need to further explore the putative link between parkin dominant toxicity and PD.
Figures


Comment in
-
Mice expressing mutant parkin exhibit hallmark features of Parkinson's disease.J Neurosci. 2009 Jun 10;29(23):7392-4. doi: 10.1523/JNEUROSCI.1719-09.2009. J Neurosci. 2009. PMID: 19515906 Free PMC article. No abstract available.
Similar articles
-
Parkin is protective for substantia nigra dopamine neurons in a tau gene transfer neurodegeneration model.Neurosci Lett. 2006 Jun 19;401(1-2):130-5. doi: 10.1016/j.neulet.2006.03.001. Epub 2006 Mar 22. Neurosci Lett. 2006. PMID: 16554120 Free PMC article.
-
LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function.Hum Mol Genet. 2016 Mar 1;25(5):951-63. doi: 10.1093/hmg/ddv628. Epub 2016 Jan 6. Hum Mol Genet. 2016. PMID: 26744332 Free PMC article.
-
A53T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration.J Neurosci. 2015 Jan 21;35(3):890-905. doi: 10.1523/JNEUROSCI.0089-14.2015. J Neurosci. 2015. PMID: 25609609 Free PMC article.
-
RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review.
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
Cited by
-
Genetic animal models of Parkinson's disease.Neuron. 2010 Jun 10;66(5):646-61. doi: 10.1016/j.neuron.2010.04.034. Neuron. 2010. PMID: 20547124 Free PMC article. Review.
-
Quantitative caveats of standard immunohistochemical procedures: implications for optical disector-based designs.J Histochem Cytochem. 2010 Jul;58(7):577-84. doi: 10.1369/jhc.2009.954164. Epub 2009 Dec 7. J Histochem Cytochem. 2010. PMID: 19995945 Free PMC article.
-
Lithium prevents parkinsonian behavioral and striatal phenotypes in an aged parkin mutant transgenic mouse model.Brain Res. 2014 Dec 3;1591:111-7. doi: 10.1016/j.brainres.2014.10.032. Epub 2014 Oct 27. Brain Res. 2014. PMID: 25452026 Free PMC article.
-
Animal Models of Autosomal Recessive Parkinsonism.Biomedicines. 2021 Jul 13;9(7):812. doi: 10.3390/biomedicines9070812. Biomedicines. 2021. PMID: 34356877 Free PMC article. Review.
-
Parkinson's disease: animal models and dopaminergic cell vulnerability.Front Neuroanat. 2014 Dec 15;8:155. doi: 10.3389/fnana.2014.00155. eCollection 2014. Front Neuroanat. 2014. PMID: 25565980 Free PMC article. Review.
References
-
- Beffert U, Rosenberg RN. Increased risk for heterozygotes in recessive Parkinson disease. Arch Neurol. 2006;63:807–808. - PubMed
-
- Binkofski F, Reetz K, Gaser C, Hilker R, Hagenah J, Hedrich K, van Eimeren T, Thiel A, Büchel C, Pramstaller PP, Siebner HR, Klein C. Morphometric fingerprint of asymptomatic Parkin and PINK1 mutation carriers in the basal ganglia. Neurology. 2007;69:842–850. - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
