Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium
- PMID: 19228882
- PMCID: PMC2696217
- DOI: 10.1152/ajpgi.00004.2009
Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium
Abstract
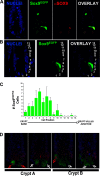
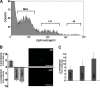
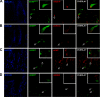
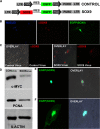
SOX transcription factors have the capacity to modulate stem/progenitor cell proliferation and differentiation in a dose-dependent manner. SOX9 is expressed in the small intestine epithelial stem cell zone. Therefore, we hypothesized that differential levels of SOX9 may exist, influencing proliferation and/or differentiation of the small intestine epithelium. Sox9 expression levels in the small intestine were investigated using a Sox9 enhanced green fluorescent protein (Sox9(EGFP)) transgenic mouse. Sox9(EGFP) levels correlate with endogenous SOX9 levels, which are expressed at two steady-state levels, termed Sox9(EGFPLO) and Sox9(EGFPHI). Crypt-based columnar cells are Sox9(EGFPLO) and demonstrate enriched expression of the stem cell marker, Lgr5. Sox9(EGFPHI) cells express chromogranin A and substance P but do not express Ki67 and neurogenin3, indicating that Sox9(EGFPHI) cells are postmitotic enteroendocrine cells. Overexpression of SOX9 in a crypt cell line stopped proliferation and induced morphological changes. These data support a bimodal role for SOX9 in the intestinal epithelium, where low SOX9 expression supports proliferative capacity, and high SOX9 expression suppresses proliferation.
Figures


Similar articles
-
Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells.Aging (Albany NY). 2017 Aug 29;9(8):1898-1915. doi: 10.18632/aging.101279. Aging (Albany NY). 2017. PMID: 28854151 Free PMC article.
-
SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine.Gastroenterology. 2015 Nov;149(6):1553-1563.e10. doi: 10.1053/j.gastro.2015.07.004. Epub 2015 Jul 11. Gastroenterology. 2015. PMID: 26170137 Free PMC article.
-
IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations.FASEB J. 2015 Jul;29(7):2828-42. doi: 10.1096/fj.14-264010. Epub 2015 Apr 2. FASEB J. 2015. PMID: 25837582 Free PMC article.
-
The role of SOX9 transcription factor in pancreatic and duodenal development.Stem Cells Dev. 2013 Nov 15;22(22):2935-43. doi: 10.1089/scd.2013.0106. Epub 2013 Aug 2. Stem Cells Dev. 2013. PMID: 23806070 Review.
-
Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways.Am J Physiol Gastrointest Liver Physiol. 2014 Aug 1;307(3):G260-73. doi: 10.1152/ajpgi.00066.2014. Epub 2014 Jun 12. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24924746 Free PMC article. Review.
Cited by
-
Development of an intestinal epithelial cell line and organoids derived from the same swine and characterization of their antiviral responses.Biosci Microbiota Food Health. 2024;43(4):342-351. doi: 10.12938/bmfh.2024-0046. Epub 2024 May 28. Biosci Microbiota Food Health. 2024. PMID: 39364127 Free PMC article.
-
Distinct roles of SOX9 in self-renewal of progenitors and mesenchymal transition of the endothelium.Angiogenesis. 2024 Aug;27(3):545-560. doi: 10.1007/s10456-024-09927-7. Epub 2024 May 11. Angiogenesis. 2024. PMID: 38733496 Free PMC article.
-
Lgr5+ intestinal stem cells are required for organoid survival after genotoxic injury.bioRxiv [Preprint]. 2024 Apr 25:2024.04.08.588400. doi: 10.1101/2024.04.08.588400. bioRxiv. 2024. PMID: 38645040 Free PMC article. Preprint.
-
Compartment specific responses to contractility in the small intestinal epithelium.PLoS Genet. 2024 Mar 22;20(3):e1010899. doi: 10.1371/journal.pgen.1010899. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38517900 Free PMC article.
-
Adult Animal Stem Cell-Derived Organoids in Biomedical Research and the One Health Paradigm.Int J Mol Sci. 2024 Jan 5;25(2):701. doi: 10.3390/ijms25020701. Int J Mol Sci. 2024. PMID: 38255775 Free PMC article. Review.
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. - PubMed
-
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160: 77–91, 1981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

