Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain
- PMID: 19226509
- PMCID: PMC2647986
- DOI: 10.1002/cne.21969
Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain
Abstract
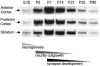
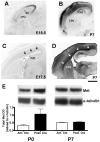
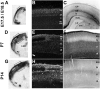
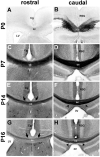
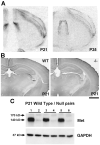
The establishment of appropriate neural circuitry depends on the coordination of multiple developmental events across space and time. These events include proliferation, migration, differentiation, and survival-all of which can be mediated by hepatocyte growth factor (HGF) signaling through the Met receptor tyrosine kinase. We previously found a functional promoter variant of the MET gene to be associated with autism spectrum disorder, suggesting that forebrain circuits governing social and emotional function may be especially vulnerable to developmental disruptions in HGF/Met signaling. However, little is known about the spatiotemporal distribution of Met expression in the forebrain during the development of such circuits. To advance our understanding of the neurodevelopmental influences of Met activation, we employed complementary Western blotting, in situ hybridization, and immunohistochemistry to comprehensively map Met transcript and protein expression throughout perinatal and postnatal development of the mouse forebrain. Our studies reveal complex and dynamic spatiotemporal patterns of expression during this period. Spatially, Met transcript is localized primarily to specific populations of projection neurons within the neocortex and in structures of the limbic system, including the amygdala, hippocampus, and septum. Met protein appears to be principally located in axon tracts. Temporally, peak expression of transcript and protein occurs during the second postnatal week. This period is characterized by extensive neurite outgrowth and synaptogenesis, supporting a role for the receptor in these processes. Collectively, these data suggest that Met signaling may be necessary for the appropriate wiring of forebrain circuits, with particular relevance to the social and emotional dimensions of behavior.
(c) 2009 Wiley-Liss, Inc.
Figures

Similar articles
-
Disruption of MET Receptor Tyrosine Kinase, an Autism Risk Factor, Impairs Developmental Synaptic Plasticity in the Hippocampus.Dev Neurobiol. 2019 Jan;79(1):36-50. doi: 10.1002/dneu.22645. Epub 2018 Oct 21. Dev Neurobiol. 2019. PMID: 30304576 Free PMC article.
-
Distinct intracellular signaling mediates C-MET regulation of dendritic growth and synaptogenesis.Dev Neurobiol. 2016 Oct;76(10):1160-81. doi: 10.1002/dneu.22382. Epub 2016 Feb 11. Dev Neurobiol. 2016. PMID: 26818605 Free PMC article.
-
Prenatal expression of MET receptor tyrosine kinase in the fetal mouse dorsal raphe nuclei and the visceral motor/sensory brainstem.Dev Neurosci. 2013;35(1):1-16. doi: 10.1159/000346367. Epub 2013 Mar 20. Dev Neurosci. 2013. PMID: 23548689 Free PMC article.
-
The Pleiotropic MET Receptor Network: Circuit Development and the Neural-Medical Interface of Autism.Biol Psychiatry. 2017 Mar 1;81(5):424-433. doi: 10.1016/j.biopsych.2016.08.035. Epub 2016 Sep 15. Biol Psychiatry. 2017. PMID: 27837921 Free PMC article. Review.
-
Lhx2, an evolutionarily conserved, multifunctional regulator of forebrain development.Brain Res. 2019 Feb 15;1705:1-14. doi: 10.1016/j.brainres.2018.02.046. Epub 2018 Mar 6. Brain Res. 2019. PMID: 29522720 Review.
Cited by
-
Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET.Toxicol Sci. 2010 Dec;118(2):625-34. doi: 10.1093/toxsci/kfq304. Epub 2010 Oct 1. Toxicol Sci. 2010. PMID: 20889680 Free PMC article.
-
Disruption of MET Receptor Tyrosine Kinase, an Autism Risk Factor, Impairs Developmental Synaptic Plasticity in the Hippocampus.Dev Neurobiol. 2019 Jan;79(1):36-50. doi: 10.1002/dneu.22645. Epub 2018 Oct 21. Dev Neurobiol. 2019. PMID: 30304576 Free PMC article.
-
Heterogeneity within Autism Spectrum Disorders: What have We Learned from Neuroimaging Studies?Front Hum Neurosci. 2013 Oct 30;7:733. doi: 10.3389/fnhum.2013.00733. Front Hum Neurosci. 2013. PMID: 24198778 Free PMC article. Review.
-
MET and AKT genetic influence on facial emotion perception.PLoS One. 2012;7(4):e36143. doi: 10.1371/journal.pone.0036143. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558359 Free PMC article.
-
Evidence for dysregulation of axonal growth and guidance in the etiology of ASD.Front Hum Neurosci. 2013 Oct 22;7:671. doi: 10.3389/fnhum.2013.00671. eCollection 2013. Front Hum Neurosci. 2013. PMID: 24155705 Free PMC article. Review.
References
-
- Aggleton JP. The amygdala : a functional analysis. xiv. Oxford University Press; Oxford, OX ; New York: 2000. p. 690.
-
- Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967;6:716–727. - PubMed
-
- Akagi K, Powell EW. Differential projections of habenular nuclei. J Comp Neurol. 1968;132:263–274. - PubMed
-
- Akimoto M, Baba A, Ikeda-Matsuo Y, Yamada MK, Itamura R, Nishiyama N, Ikegaya Y, Matsuki N. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128:155–162. - PubMed
-
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol. 1989;286:311–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

