Harmonic oscillator model of the insulin and IGF1 receptors' allosteric binding and activation
- PMID: 19225456
- PMCID: PMC2657531
- DOI: 10.1038/msb.2008.78
Harmonic oscillator model of the insulin and IGF1 receptors' allosteric binding and activation
Abstract
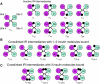
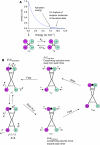
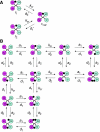
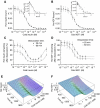
The insulin and insulin-like growth factor 1 receptors activate overlapping signalling pathways that are critical for growth, metabolism, survival and longevity. Their mechanism of ligand binding and activation displays complex allosteric properties, which no mathematical model has been able to account for. Modelling these receptors' binding and activation in terms of interactions between the molecular components is problematical due to many unknown biochemical and structural details. Moreover, substantial combinatorial complexity originating from multivalent ligand binding further complicates the problem. On the basis of the available structural and biochemical information, we develop a physically plausible model of the receptor binding and activation, which is based on the concept of a harmonic oscillator. Modelling a network of interactions among all possible receptor intermediaries arising in the context of the model (35, for the insulin receptor) accurately reproduces for the first time all the kinetic properties of the receptor, and provides unique and robust estimates of the kinetic parameters. The harmonic oscillator model may be adaptable for many other dimeric/dimerizing receptor tyrosine kinases, cytokine receptors and G-protein-coupled receptors where ligand crosslinking occurs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Solution structure of ectodomains of the insulin receptor family: the ectodomain of the type 1 insulin-like growth factor receptor displays asymmetry of ligand binding accompanied by limited conformational change.J Mol Biol. 2009 Dec 18;394(5):878-92. doi: 10.1016/j.jmb.2009.10.011. Epub 2009 Oct 14. J Mol Biol. 2009. PMID: 19835884
-
Role of the time factor in signaling specificity: application to mitogenic and metabolic signaling by the insulin and insulin-like growth factor-I receptor tyrosine kinases.Metabolism. 1995 Oct;44(10 Suppl 4):2-11. doi: 10.1016/0026-0495(95)90214-7. Metabolism. 1995. PMID: 7476307 Review.
-
Identification of common ligand binding determinants of the insulin and insulin-like growth factor 1 receptors. Insights into mechanisms of ligand binding.J Biol Chem. 1997 Jul 25;272(30):18650-5. doi: 10.1074/jbc.272.30.18650. J Biol Chem. 1997. PMID: 9228034
-
The insulin receptor: a prototype for dimeric, allosteric membrane receptors?Trends Biochem Sci. 2008 Aug;33(8):376-84. doi: 10.1016/j.tibs.2008.06.003. Epub 2008 Jul 18. Trends Biochem Sci. 2008. PMID: 18640841 Review.
-
The insulin receptor changes conformation in unforeseen ways on ligand binding: sharpening the picture of insulin receptor activation.Bioessays. 2013 Nov;35(11):945-54, doi/10.1002/bies.201370111. doi: 10.1002/bies.201300065. Epub 2013 Aug 28. Bioessays. 2013. PMID: 24037759 Review.
Cited by
-
Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights.Int J Mol Sci. 2020 Jul 2;21(13):4725. doi: 10.3390/ijms21134725. Int J Mol Sci. 2020. PMID: 32630698 Free PMC article. Review.
-
Mass and information feedbacks through receptor endocytosis govern insulin signaling as revealed using a parameter-free modeling framework.J Biol Chem. 2010 Jun 25;285(26):20171-9. doi: 10.1074/jbc.M110.106849. Epub 2010 Apr 26. J Biol Chem. 2010. PMID: 20421297 Free PMC article.
-
Structural basis of the activation of type 1 insulin-like growth factor receptor.Nat Commun. 2019 Oct 8;10(1):4567. doi: 10.1038/s41467-019-12564-0. Nat Commun. 2019. PMID: 31594955 Free PMC article.
-
Quasi-Steady-State Analysis based on Structural Modules and Timed Petri Net Predict System's Dynamics: The Life Cycle of the Insulin Receptor.Metabolites. 2015 Dec 17;5(4):766-93. doi: 10.3390/metabo5040766. Metabolites. 2015. PMID: 26694479 Free PMC article.
-
The Role of Catechins in Regulating Diabetes: An Update Review.Nutrients. 2022 Nov 4;14(21):4681. doi: 10.3390/nu14214681. Nutrients. 2022. PMID: 36364943 Free PMC article. Review.
References
-
- Bloch SC (1997) Introduction to Classical and Quantum Harmonic Oscillators. New York: John Wiley & Sons Inc.
-
- Brzozowski AM, Dodson EJ, Dodson GG, Murshudov GN, Verma C, Turkenburg JP, de Bree FM, Dauter Z (2002) Structural origins of the functional divergence of human insulin-like growth factor-I and insulin. Biochemistry 41: 9389–9397 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

