Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors
- PMID: 19224531
- PMCID: PMC3014988
- DOI: 10.1002/ana.21539
Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors
Abstract
Objective: Glutamate receptors, which play a major role in the physiology and pathology of central nervous system gray matter, are also involved in the pathophysiology of white matter. However, the cellular and molecular mechanisms responsible for excitotoxic damage to white matter elements are not fully understood. We explored the roles of AMPA and GluR5 kainate receptors in axonal Ca(2+) deregulation.
Methods: Dorsal column axons were loaded with a Ca(2+) indicator and imaged in vitro using confocal microscopy.
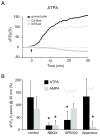
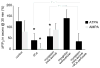
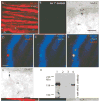
Results: Both AMPA and a GluR5 kainate receptor agonist increased intraaxonal Ca(2+) in myelinated rat dorsal column fibers. These responses were inhibited by selective antagonists of these receptors. The GluR5-mediated Ca(2+) increase was mediated by both canonical (ie, ionotropic) and noncanonical (metabotropic) signaling, dependent on a pertussis toxin-sensitive G protein/phospholipase C-dependent pathway, promoting Ca(2+) release from inositol triphosphate-dependent stores. In addition, the GluR5 response was reduced by intraaxonal NO scavengers. In contrast, GluR4 AMPA receptors operated via Ca(2+)-induced Ca(2+) release, dependent on ryanodine receptors, and unaffected by NO scavengers. Neither pathway depended on L-type Ca(2+) channels, in contrast with GluR6 kainate receptor action.1 Immunohistochemistry confirmed the presence of GluR4 and GluR5 clustered at the surface of myelinated axons; GluR5 coimmunoprecipitated with nNOS and often colocalized with neuronal nitric oxide synthase clusters on the internodal axon.
Interpretation: Central myelinated axons express functional AMPA and GluR5 kainate receptors, and can directly respond to glutamate receptor agonists. These glutamate receptor-dependent signaling pathways promote an increase in intraaxonal Ca(2+) levels potentially contributing to axonal degeneration.
Figures
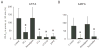
Comment in
-
Axons get excited to death.Ann Neurol. 2009 Feb;65(2):120-1. doi: 10.1002/ana.21659. Ann Neurol. 2009. PMID: 19259963 Free PMC article. No abstract available.
Similar articles
-
Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors.Ann Neurol. 2009 Feb;65(2):151-9. doi: 10.1002/ana.21533. Ann Neurol. 2009. PMID: 19224535 Free PMC article.
-
Na(+)-K(+)-ATPase inhibition and depolarization induce glutamate release via reverse Na(+)-dependent transport in spinal cord white matter.Neuroscience. 2001;107(4):675-83. doi: 10.1016/s0306-4522(01)00385-2. Neuroscience. 2001. PMID: 11720790
-
Overactivation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate and N-methyl-D-aspartate but not kainate receptors inhibits phosphatidylcholine synthesis before excitotoxic neuronal death.J Neurochem. 2001 Apr;77(1):13-22. doi: 10.1046/j.1471-4159.2001.t01-2-00187.x. J Neurochem. 2001. PMID: 11279257
-
New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors.Pharmacol Ther. 1996;70(1):65-89. doi: 10.1016/0163-7258(96)00014-9. Pharmacol Ther. 1996. PMID: 8804111 Review.
-
Diversity of AMPA Receptor Ligands: Chemotypes, Binding Modes, Mechanisms of Action, and Therapeutic Effects.Biomolecules. 2022 Dec 27;13(1):56. doi: 10.3390/biom13010056. Biomolecules. 2022. PMID: 36671441 Free PMC article. Review.
Cited by
-
Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors.Ann Neurol. 2009 Feb;65(2):151-9. doi: 10.1002/ana.21533. Ann Neurol. 2009. PMID: 19224535 Free PMC article.
-
Axons get excited to death.Ann Neurol. 2009 Feb;65(2):120-1. doi: 10.1002/ana.21659. Ann Neurol. 2009. PMID: 19259963 Free PMC article. No abstract available.
-
Soluble Tumor Necrosis Factor Alpha Promotes Retinal Ganglion Cell Death in Glaucoma via Calcium-Permeable AMPA Receptor Activation.J Neurosci. 2015 Sep 2;35(35):12088-102. doi: 10.1523/JNEUROSCI.1273-15.2015. J Neurosci. 2015. PMID: 26338321 Free PMC article.
-
Age-related changes of myelin basic protein in mouse and human auditory nerve.PLoS One. 2012;7(4):e34500. doi: 10.1371/journal.pone.0034500. Epub 2012 Apr 5. PLoS One. 2012. PMID: 22496821 Free PMC article.
-
Galvanic vestibular stimulation down-regulated NMDA receptors in vestibular nucleus of PD model.Sci Rep. 2022 Nov 8;12(1):18999. doi: 10.1038/s41598-022-20876-3. Sci Rep. 2022. PMID: 36347898 Free PMC article.
References
-
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. - PubMed
-
- Park E, Liu Y, Fehlings MG. Changes in glial cell white matter AMPA receptor expression after spinal cord injury and relationship to apoptotic cell death. Exp Neurol. 2003;182:35–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

