HIF-1alpha determines the metastatic potential of gastric cancer cells
- PMID: 19223895
- PMCID: PMC2653758
- DOI: 10.1038/sj.bjc.6604919
HIF-1alpha determines the metastatic potential of gastric cancer cells
Abstract
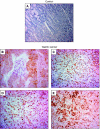
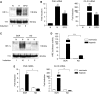
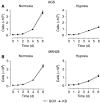
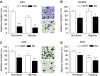
Gastric adenocarcinoma is characterised by rapid emergence of systemic metastases, resulting in poor prognosis due to vanished curative treatment options. Better understanding of the molecular basis of gastric cancer spread is needed to design innovative treatments. The transcription factor HIF-1alpha (hypoxia-inducible factor 1alpha) is frequently overexpressed in human gastric cancer, and inhibition of HIF-1alpha has proven antitumour efficacy in rodent models, whereas the relevance of HIF-1alpha for the metastatic phenotype of gastric adenocarcinoma remains elusive. Therefore, we have conducted a comprehensive analysis of the role of HIF-1alpha for pivotal metastasis-associated processes of human gastric cancer. Immunhistochemistry for HIF-1alpha showed specific staining at the invading tumour edge in 90% of human gastric cancer samples, whereas normal gastric tissue was negative and only a minority of early gastric cancers (T1 tumours) showed specific staining. Hypoxia-inducible factor 1alpha-deficient cells showed a significant reduction of migratory, invasive and adhesive properties in vitro. Furthermore, the HIF-1alpha-inhibitor 2-methoxy-estradiol significantly reduced metastatic properties of gastric cancer cells. The accentuated expression at the invading edge together with the in vitro requirement of HIF-1alpha for migration, invasion and adherence argues for a pivotal role of HIF-1alpha in local invasion and, ultimately, systemic tumour spread. These results warrant the exploration of HIF-1alpha-inhibiting substances in clinical treatment studies of advanced gastric cancer.
Figures

Similar articles
-
Upregulation of hypoxia inducible factor 1alpha mRNA is associated with elevated vascular endothelial growth factor expression and excessive angiogenesis and predicts a poor prognosis in gastric carcinoma.World J Gastroenterol. 2007 Mar 21;13(11):1680-6. doi: 10.3748/wjg.v13.i11.1680. World J Gastroenterol. 2007. PMID: 17461470 Free PMC article.
-
Down-regulation of HIF-1α inhibits the proliferation, migration, and invasion of gastric cancer by inhibiting PI3K/AKT pathway and VEGF expression.Biosci Rep. 2018 Dec 21;38(6):BSR20180741. doi: 10.1042/BSR20180741. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 29899167 Free PMC article.
-
HIF-1α is a crucial factor in the development of peritoneal dissemination via natural metastatic routes in scirrhous gastric cancer.Int J Oncol. 2013 Nov;43(5):1431-40. doi: 10.3892/ijo.2013.2068. Epub 2013 Aug 21. Int J Oncol. 2013. PMID: 23970191
-
Prognostic value of HIF-1α expression in patients with gastric cancer.Mol Biol Rep. 2013 Nov;40(11):6055-62. doi: 10.1007/s11033-013-2715-z. Epub 2013 Sep 23. Mol Biol Rep. 2013. PMID: 24057269 Review.
-
Is the hypoxia-inducible factor pathway important in gastric cancer?Eur J Cancer. 2005 Dec;41(18):2792-805. doi: 10.1016/j.ejca.2005.09.008. Epub 2005 Nov 14. Eur J Cancer. 2005. PMID: 16290133 Review.
Cited by
-
Lentivirus-mediated RASSF1A expression suppresses aggressive phenotypes of gastric cancer cells in vitro and in vivo.Gene Ther. 2015 Oct;22(10):793-801. doi: 10.1038/gt.2015.49. Epub 2015 May 25. Gene Ther. 2015. PMID: 26005859 Free PMC article.
-
Role of hypoxia-inducible transcription factor 1alpha for progression and chemosensitivity of murine hepatocellular carcinoma.J Mol Med (Berl). 2010 Aug;88(8):817-27. doi: 10.1007/s00109-010-0623-4. Epub 2010 Apr 12. J Mol Med (Berl). 2010. PMID: 20383692
-
Relationship between Volatile Anesthetics and Tumor Progression: Unveiling the Mystery.Curr Med Sci. 2018 Dec;38(6):962-967. doi: 10.1007/s11596-018-1970-6. Epub 2018 Dec 7. Curr Med Sci. 2018. PMID: 30536056 Review.
-
Hypoxia Induced ER Stress Response as an Adaptive Mechanism in Cancer.Int J Mol Sci. 2019 Feb 11;20(3):749. doi: 10.3390/ijms20030749. Int J Mol Sci. 2019. PMID: 30754624 Free PMC article. Review.
-
Modulation of urokinase plasminogen activator system by poly(ADP-ribose)polymerase-1 inhibition.Cytotechnology. 2016 Aug;68(4):783-94. doi: 10.1007/s10616-014-9829-6. Epub 2014 Dec 4. Cytotechnology. 2016. PMID: 25471275 Free PMC article.
References
-
- Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M (2001) Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res 61: 6548–6554 - PubMed
-
- Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G (2000) Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res 60: 4693–4696 - PubMed
-
- Blancher C, Moore JW, Robertson N, Harris AL (2001) Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res 61: 7349–7355 - PubMed
-
- Bogenrieder T, Herlyn M (2003) Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 22: 6524–6536 - PubMed
-
- Bos R, van der GP, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der WE (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97: 1573–1581 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

