Contribution of oxidative damage to antimicrobial lethality
- PMID: 19223646
- PMCID: PMC2663088
- DOI: 10.1128/AAC.01087-08
Contribution of oxidative damage to antimicrobial lethality
Abstract
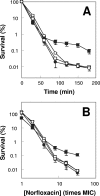
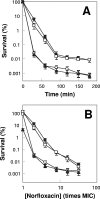
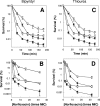
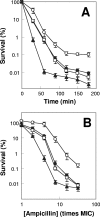
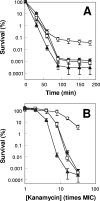
A potential pathway linking hydroxyl radicals to antimicrobial lethality was examined by using mutational and chemical perturbations of Escherichia coli. Deficiencies of sodA or sodB had no effect on norfloxacin lethality; however, the absence of both genes together reduced lethal activity, consistent with rapid conversion of excessive superoxide to hydrogen peroxide contributing to quinolone lethality. Norfloxacin was more lethal with a mutant deficient in katG than with its isogenic parent, suggesting that detoxification of peroxide to water normally reduces quinolone lethality. An iron chelator (bipyridyl) and a hydroxyl radical scavenger (thiourea) reduced the lethal activity of norfloxacin, indicating that norfloxacin-stimulated accumulation of peroxide affects lethal activity via hydroxyl radicals generated through the Fenton reaction. Ampicillin and kanamycin, antibacterials unrelated to fluoroquinolones, displayed behavior similar to that of norfloxacin except that these two agents showed hyperlethality with an ahpC (alkyl hydroperoxide reductase) mutant rather than with a katG mutant. Collectively, these data are consistent with antimicrobial stress increasing the production of superoxide, which then undergoes dismutation to peroxide, from which a highly toxic hydroxyl radical is generated. Hydroxyl radicals then enhance antimicrobial lethality, as suggested by earlier work. Such findings indicate that oxidative stress networks may provide targets for antimicrobial potentiation.
Figures
Similar articles
-
[Role of reactive oxygen species in the bactericidal action of quinolones--inhibitors of DNA gyrase].Mol Biol (Mosk). 2014 Nov-Dec;48(6):990-8. Mol Biol (Mosk). 2014. PMID: 25845240 Russian.
-
A common mechanism of cellular death induced by bactericidal antibiotics.Cell. 2007 Sep 7;130(5):797-810. doi: 10.1016/j.cell.2007.06.049. Cell. 2007. PMID: 17803904
-
Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death.J Antimicrob Chemother. 2010 Mar;65(3):520-4. doi: 10.1093/jac/dkp486. Epub 2010 Jan 12. J Antimicrob Chemother. 2010. PMID: 20067982 Free PMC article.
-
Hydroxyl radicals are involved in cell killing by the bacterial topoisomerase I cleavage complex.J Bacteriol. 2009 Aug;191(16):5315-9. doi: 10.1128/JB.00559-09. Epub 2009 Jun 12. J Bacteriol. 2009. PMID: 19525344 Free PMC article.
-
[Free oxygen radiacals and kidney diseases--part I].Med Pregl. 2000 Sep-Oct;53(9-10):463-74. Med Pregl. 2000. PMID: 11320727 Review. Croatian.
Cited by
-
Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells.Sci Transl Med. 2013 Jul 3;5(192):192ra85. doi: 10.1126/scitranslmed.3006055. Sci Transl Med. 2013. PMID: 23825301 Free PMC article.
-
YihE kinase is a central regulator of programmed cell death in bacteria.Cell Rep. 2013 Feb 21;3(2):528-37. doi: 10.1016/j.celrep.2013.01.026. Epub 2013 Feb 14. Cell Rep. 2013. PMID: 23416055 Free PMC article.
-
Antibiotic and ROS linkage questioned.Nat Biotechnol. 2013 May;31(5):415-6. doi: 10.1038/nbt.2574. Nat Biotechnol. 2013. PMID: 23657395 No abstract available.
-
Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species.J Biol Chem. 2010 Jul 16;285(29):22689-95. doi: 10.1074/jbc.M110.127456. Epub 2010 May 17. J Biol Chem. 2010. PMID: 20479007 Free PMC article.
-
Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli.Antimicrob Agents Chemother. 2011 Mar;55(3):1053-62. doi: 10.1128/AAC.01002-10. Epub 2011 Jan 3. Antimicrob Agents Chemother. 2011. PMID: 21199923 Free PMC article.
References
-
- Albesa, I., M. C. Becerra, P. C. Battan, and P. L. Paez. 2004. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 317:605-609. - PubMed
-
- Amstad, P., R. Moret, and P. Cerutti. 1994. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J. Biol. Chem. 269:1606-1609. - PubMed
-
- Amstad, P., A. Peskin, G. Shah, M. E. Mirault, R. Moret, I. Zbinden, and P. Cerutti. 1991. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry 30:9305-9313. - PubMed
-
- Becerra, M. C., and I. Albesa. 2002. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem. Biophys. Res. Commun. 297:1003-1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

