Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation
- PMID: 19218437
- PMCID: PMC2651292
- DOI: 10.1073/pnas.0810169106
Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation
Abstract
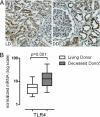
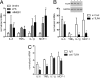
While studies in animal models have linked Toll-like receptor (TLR) 4 signaling to kidney injury induced by ischemia and reperfusion, the relevance of TLR4 activation to allograft injury in human kidney transplants is unknown. Here we show that TLR4 is constitutively expressed within all donor kidneys but is significantly higher in deceased-, compared with living-donor organs. Tubules from deceased- but not living-donor kidneys also stained positively for high-mobility group box-1 (HMGB1), a known endogenous TLR4 ligand. In vitro stimulation of human tubular cells with HMGB1, in a TLR4-dependent system, confirmed that HMGB1 can stimulate proinflammatory responses through TLR4. To assess the functional significance of TLR4 in human kidney transplantation, we determined whether TLR4 mutations that confer diminished affinity for HMGB1 influence intragraft gene-expression profiles and immediate graft function. Compared with kidneys expressing WT alleles, kidneys with a TLR4 loss-of-function allele contained less TNFalpha, MCP-1, and more heme oxygenase 1 (HO-1), and exhibited a higher rate of immediate graft function. These results represent previously undetected evidence that donor TLR4 contributes to graft inflammation and sterile injury following cold preservation and transplantation in humans. Targeting TLR4 signaling may have value in preventing or treating postischemic acute kidney injury after transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
HMGB1 contributes to kidney ischemia reperfusion injury.J Am Soc Nephrol. 2010 Nov;21(11):1878-90. doi: 10.1681/ASN.2009101048. Epub 2010 Sep 16. J Am Soc Nephrol. 2010. PMID: 20847143 Free PMC article.
-
A single nucleotide polymorphism of Toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation.J Hepatol. 2010 Jul;53(1):67-72. doi: 10.1016/j.jhep.2009.12.044. Epub 2010 Mar 30. J Hepatol. 2010. PMID: 20400193
-
Acetyl-3-Aminoethyl Salicylate Ameliorates Hepatic Ischemia/Reperfusion Injury and Liver Graft Survival Through a High-Mobility Group Box 1/Toll-Like Receptor 4-Dependent Mechanism.Liver Transpl. 2019 Aug;25(8):1220-1232. doi: 10.1002/lt.25575. Epub 2019 Jul 8. Liver Transpl. 2019. PMID: 31125492
-
Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2014 Apr 15;306(8):F801-11. doi: 10.1152/ajprenal.00469.2013. Epub 2014 Feb 12. Am J Physiol Renal Physiol. 2014. PMID: 24523386 Free PMC article. Review.
-
Acute kidney injury: a conspiracy of Toll-like receptor 4 on endothelia, leukocytes, and tubules.Pediatr Nephrol. 2012 Oct;27(10):1847-54. doi: 10.1007/s00467-011-2029-0. Epub 2011 Oct 28. Pediatr Nephrol. 2012. PMID: 22033798 Free PMC article. Review.
Cited by
-
In the face of chronic aspiration, prolonged ischemic time exacerbates obliterative bronchiolitis in rat pulmonary allografts.Am J Transplant. 2012 Nov;12(11):2930-7. doi: 10.1111/j.1600-6143.2012.04215.x. Epub 2012 Aug 6. Am J Transplant. 2012. PMID: 22882880 Free PMC article.
-
High-mobility group box 1 inhibits HCO(3)(-) absorption in medullary thick ascending limb through a basolateral receptor for advanced glycation end products pathway.Am J Physiol Renal Physiol. 2015 Oct 15;309(8):F720-30. doi: 10.1152/ajprenal.00227.2015. Epub 2015 Jul 15. Am J Physiol Renal Physiol. 2015. PMID: 26180239 Free PMC article.
-
Genetic susceptibility to delayed graft function following kidney transplantation: a systematic review of the literature.Clin Kidney J. 2018 Aug;11(4):586-596. doi: 10.1093/ckj/sfy020. Epub 2018 Apr 3. Clin Kidney J. 2018. PMID: 30090630 Free PMC article.
-
Hypoxia and hyperbaric oxygen therapy: a review.Int J Gen Med. 2018 Nov 20;11:431-442. doi: 10.2147/IJGM.S172460. eCollection 2018. Int J Gen Med. 2018. PMID: 30538529 Free PMC article. Review.
-
The long noncoding RNA Meg3 mediates TLR4-induced inflammation in experimental obstructive nephropathy.Clin Sci (Lond). 2023 Mar 15;137(5):317-331. doi: 10.1042/CS20220537. Clin Sci (Lond). 2023. PMID: 36705251 Free PMC article.
References
-
- Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814–1827. - PubMed
-
- Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66:1697–1701. - PubMed
-
- Yokoyama I, et al. Effect of prolonged delayed graft function on long-term graft outcome in cadaveric kidney transplantation. Clin Transplant. 1994;8:101–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

