The Toca-1-N-WASP complex links filopodial formation to endocytosis
- PMID: 19213734
- PMCID: PMC2670167
- DOI: 10.1074/jbc.M805940200
The Toca-1-N-WASP complex links filopodial formation to endocytosis
Abstract
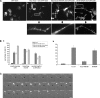
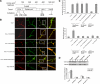
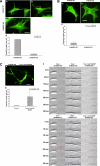
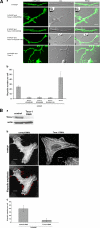
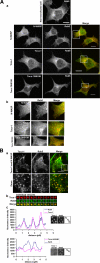
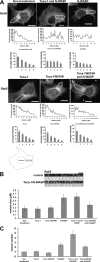
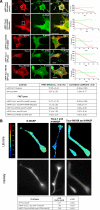
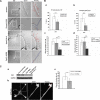
The transducer of Cdc42-dependent actin assembly (Toca-1)-N-WASP complex was isolated as an essential cofactor for Cdc42-driven actin polymerization in vitro. Toca-1 consists of an N-terminal F-BAR domain, followed by a Cdc42 binding site (HR1 domain) and an SH3 domain, (the N-WASP interacting site). N-WASP is an activator of actin nucleation through the Arp2/3 complex. The aim of the present study was to investigate the cellular function of the Toca-1-N-WASP complex. We report that Toca-1 induces filopodia and neurites as does N-WASP in N1E115 neuroblastoma cells. Toca-1 requires the F-BAR domain, Cdc42 binding site, and SH3 domain to induce filopodia. Toca-1 and N-WASP both require each other to induce filopodia. The expression of Toca-1 and N-WASP affects the distribution, size, and number of Rab5 positive membranes. Toca-1 interacts directly with N-WASP in filopodia and Rab5 membrane as seen by Forster resonance energy transfer. Thus the Toca-1-N-WASP complex localizes to and induces the formation of filopodia and endocytic vesicles. Last, three inhibitors of endocytosis, Dynamin-K44A, Eps15Delta95/295, and clathrin heavy chain RNA interference, block Toca-1-induced filopodial formation. Taken together, these data suggest that the Toca-1-N-WASP complex can link filopodial formation to endocytosis.
Figures

Similar articles
-
Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis.PLoS One. 2010 Aug 13;5(8):e12153. doi: 10.1371/journal.pone.0012153. PLoS One. 2010. PMID: 20730103 Free PMC article.
-
EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization.EMBO J. 2008 Nov 5;27(21):2817-28. doi: 10.1038/emboj.2008.216. Epub 2008 Oct 16. EMBO J. 2008. PMID: 18923421 Free PMC article.
-
Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination.J Biol Chem. 2006 Sep 29;281(39):29042-53. doi: 10.1074/jbc.M604025200. Epub 2006 Aug 2. J Biol Chem. 2006. PMID: 16885158
-
Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover.Small GTPases. 2017 Oct 2;8(4):237-244. doi: 10.1080/21541248.2016.1215657. Epub 2016 Aug 11. Small GTPases. 2017. PMID: 27715449 Free PMC article. Review.
-
I-BAR domains, IRSp53 and filopodium formation.Semin Cell Dev Biol. 2010 Jun;21(4):350-6. doi: 10.1016/j.semcdb.2009.11.008. Epub 2009 Nov 11. Semin Cell Dev Biol. 2010. PMID: 19913105 Review.
Cited by
-
toca-1 is in a novel pathway that functions in parallel with a SUN-KASH nuclear envelope bridge to move nuclei in Caenorhabditis elegans.Genetics. 2013 Jan;193(1):187-200. doi: 10.1534/genetics.112.146589. Epub 2012 Nov 12. Genetics. 2013. PMID: 23150597 Free PMC article.
-
Rab5 and Rab4 regulate axon elongation in the Xenopus visual system.J Neurosci. 2014 Jan 8;34(2):373-91. doi: 10.1523/JNEUROSCI.0876-13.2014. J Neurosci. 2014. PMID: 24403139 Free PMC article.
-
Dynamin1 is a novel target for IRSp53 protein and works with mammalian enabled (Mena) protein and Eps8 to regulate filopodial dynamics.J Biol Chem. 2014 Aug 29;289(35):24383-96. doi: 10.1074/jbc.M114.553883. Epub 2014 Jul 16. J Biol Chem. 2014. PMID: 25031323 Free PMC article.
-
F-BAR domain proteins: Families and function.Commun Integr Biol. 2010 Mar;3(2):116-21. doi: 10.4161/cib.3.2.10808. Commun Integr Biol. 2010. PMID: 20585502 Free PMC article.
-
N-WASP coordinates the delivery and F-actin-mediated capture of MT1-MMP at invasive pseudopods.J Cell Biol. 2012 Oct 29;199(3):527-44. doi: 10.1083/jcb.201203025. Epub 2012 Oct 22. J Cell Biol. 2012. PMID: 23091069 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

