Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology
- PMID: 19211891
- PMCID: PMC2699573
- DOI: 10.1523/JNEUROSCI.5715-08.2009
Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology
Abstract
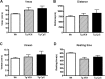
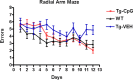
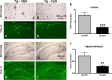
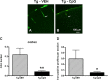
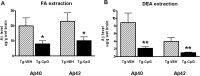
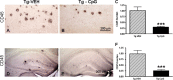
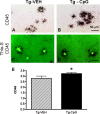
The pathogenesis of Alzheimer's disease (AD) is thought to be related to the accumulation of amyloid beta (Abeta) in amyloid deposits and toxic oligomeric species. Immunomodulation is emerging as an effective means of shifting the equilibrium from Abeta accumulation to clearance; however, excessive cell mediated inflammation and cerebral microhemorrhages are two forms of toxicity which can occur with this approach. Vaccination studies have so far mainly targeted the adaptive immune system. In the present study, we have stimulated the innate immune system via the Toll-like receptor 9 (TLR9) with cytosine-guanosine-containing DNA oligodeoxynucleotides in Tg2576 AD model transgenic mice. This treatment produced a 66% and 80% reduction in the cortical (p = 0.0001) and vascular (p = 0.0039) amyloid burden, respectively, compared with nontreated AD mice. This was in association with significant reductions in Abeta42, Abeta40, and Abeta oligomer levels. We also show that treated Tg mice performed similarly to wild-type mice on a radial arm maze. Our data suggest that stimulation of innate immunity via TLR9 is highly effective at reducing the parenchymal and vascular amyloid burden, along with Abeta oligomers, without apparent toxicity.
Figures




Similar articles
-
Innate Immunity Stimulation via Toll-Like Receptor 9 Ameliorates Vascular Amyloid Pathology in Tg-SwDI Mice with Associated Cognitive Benefits.J Neurosci. 2017 Jan 25;37(4):936-959. doi: 10.1523/JNEUROSCI.1967-16.2016. J Neurosci. 2017. PMID: 28123027 Free PMC article.
-
Amyloid β and Tau Alzheimer's disease related pathology is reduced by Toll-like receptor 9 stimulation.Acta Neuropathol Commun. 2014 Sep 2;2:101. doi: 10.1186/s40478-014-0101-2. Acta Neuropathol Commun. 2014. PMID: 25178404 Free PMC article.
-
Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages.Eur J Neurosci. 2006 Nov;24(9):2530-42. doi: 10.1111/j.1460-9568.2006.05149.x. Eur J Neurosci. 2006. PMID: 17100841 Free PMC article.
-
Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease.J Neurosci. 2001 Jan 15;21(2):372-81. doi: 10.1523/JNEUROSCI.21-02-00372.2001. J Neurosci. 2001. PMID: 11160418 Free PMC article.
-
Knockout of apolipoprotein A-I decreases parenchymal and vascular β-amyloid pathology in the Tg2576 mouse model of Alzheimer's disease.Neuropathol Appl Neurobiol. 2019 Dec;45(7):698-714. doi: 10.1111/nan.12556. Epub 2019 May 14. Neuropathol Appl Neurobiol. 2019. PMID: 31002190
Cited by
-
Microglial modulation as a therapeutic strategy in Alzheimer's disease: Focus on microglial preconditioning approaches.J Cell Mol Med. 2024 Aug;28(15):e18554. doi: 10.1111/jcmm.18554. J Cell Mol Med. 2024. PMID: 39103747 Free PMC article. Review.
-
Modulation of Alzheimer's disease brain pathology in mice by gut bacterial depletion: the role of IL-17a.Gut Microbes. 2024 Jan-Dec;16(1):2363014. doi: 10.1080/19490976.2024.2363014. Epub 2024 Jun 21. Gut Microbes. 2024. PMID: 38904096 Free PMC article.
-
Astrocyte-specific knockout of YKL-40/Chi3l1 reduces Aβ burden and restores memory functions in 5xFAD mice.J Neuroinflammation. 2023 Dec 2;20(1):290. doi: 10.1186/s12974-023-02970-z. J Neuroinflammation. 2023. PMID: 38042775 Free PMC article.
-
Mitochondrial DNA and Inflammation in Alzheimer's Disease.Curr Issues Mol Biol. 2023 Oct 25;45(11):8586-8606. doi: 10.3390/cimb45110540. Curr Issues Mol Biol. 2023. PMID: 37998717 Free PMC article. Review.
-
Inhibition of cGAS-STING pathway alleviates neuroinflammation-induced retinal ganglion cell death after ischemia/reperfusion injury.Cell Death Dis. 2023 Sep 19;14(9):615. doi: 10.1038/s41419-023-06140-0. Cell Death Dis. 2023. PMID: 37726272 Free PMC article.
References
-
- Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Aβ derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer's model mice. Eur J Neurosci. 2006;24:2530–2542. - PMC - PubMed
-
- Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. - PubMed
-
- Bombois S, Maurage CA, Gompel M, Deramecourt V, Mackowiak-Cordoliani MA, Black RS, Lavielle R, Delacourte A, Pasquier F. Absence of beta-amyloid deposits after immunization in Alzheimer disease with Lewy body dementia. Arch Neurol. 2007;64:583–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
