Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases
- PMID: 19208908
- PMCID: PMC2671039
- DOI: 10.2337/db08-1536
Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases
Abstract
Objective: We investigated the role of cytochrome P450 of the 4A family (CYP4A), its metabolites, and NADPH oxidases both in reactive oxygen species (ROS) production and apoptosis of podocytes exposed to high glucose and in OVE26 mice, a model of type 1 diabetes.
Research design and methods: Apoptosis, albuminuria, ROS generation, NADPH superoxide generation, CYP4A and Nox protein expression, and mRNA levels were measured in vitro and in vivo.
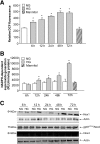
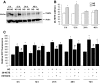
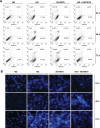
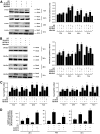
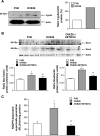
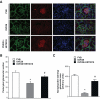
Results: Exposure of mouse podocytes to high glucose resulted in apoptosis, with approximately one-third of the cells being apoptotic by 72 h. High-glucose treatment increased ROS generation and was associated with sequential upregulation of CYP4A and an increase in 20-hydroxyeicosatetraenoic acid (20-HETE) and Nox oxidases. This is consistent with the observation of delayed induction of NADPH oxidase activity by high glucose. The effects of high glucose on NADPH oxidase activity, Nox proteins and mRNA expression, and apoptosis were blocked by N-hydroxy-N'-(4-butyl-2-methylphenol) formamidine (HET0016), an inhibitor of CYP4A, and were mimicked by 20-HETE. CYP4A and Nox oxidase expression was upregulated in glomeruli of type 1 diabetic OVE26 mice. Treatment of OVE26 mice with HET0016 decreased NADPH oxidase activity and Nox1 and Nox4 protein expression and ameliorated apoptosis and albuminuria.
Conclusions: Generation of ROS by CYP4A monooxygenases, 20-HETE, and Nox oxidases is involved in podocyte apoptosis in vitro and in vivo. Inhibition of selected cytochrome P450 isoforms prevented podocyte apoptosis and reduced proteinuria in diabetes.
Figures


Similar articles
-
Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes.Am J Physiol Renal Physiol. 2015 Jun 1;308(11):F1276-87. doi: 10.1152/ajprenal.00396.2014. Epub 2015 Feb 4. Am J Physiol Renal Physiol. 2015. PMID: 25656366 Free PMC article.
-
The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo.Am J Physiol Renal Physiol. 2013 Sep 1;305(5):F691-700. doi: 10.1152/ajprenal.00028.2013. Epub 2013 Jun 26. Am J Physiol Renal Physiol. 2013. PMID: 23804455 Free PMC article.
-
mTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes.Antioxid Redox Signal. 2016 Nov 1;25(13):703-719. doi: 10.1089/ars.2015.6562. Epub 2016 Sep 12. Antioxid Redox Signal. 2016. PMID: 27393154 Free PMC article.
-
The contribution of TRPV1 channel to 20-HETE-Aggravated ischemic neuronal injury.Prostaglandins Other Lipid Mediat. 2018 Jul;137:63-68. doi: 10.1016/j.prostaglandins.2018.07.001. Epub 2018 Jul 6. Prostaglandins Other Lipid Mediat. 2018. PMID: 30041768 Free PMC article. Review.
-
Role of the CYP4A/20-HETE pathway in vascular dysfunction of the Dahl salt-sensitive rat.Clin Sci (Lond). 2013 Jun;124(12):695-700. doi: 10.1042/CS20120483. Clin Sci (Lond). 2013. PMID: 23438293 Free PMC article. Review.
Cited by
-
Cross talk between ceramide and redox signaling: implications for endothelial dysfunction and renal disease.Handb Exp Pharmacol. 2013;(216):171-97. doi: 10.1007/978-3-7091-1511-4_9. Handb Exp Pharmacol. 2013. PMID: 23563657 Free PMC article. Review.
-
Contribution of cytochrome P450 1B1 to hypertension and associated pathophysiology: a novel target for antihypertensive agents.Prostaglandins Other Lipid Mediat. 2012 Aug;98(3-4):69-74. doi: 10.1016/j.prostaglandins.2011.12.003. Epub 2011 Dec 20. Prostaglandins Other Lipid Mediat. 2012. PMID: 22210049 Free PMC article. Review.
-
The roles of hydrogen sulfide in renal physiology and disease states.Ren Fail. 2022 Dec;44(1):1289-1308. doi: 10.1080/0886022X.2022.2107936. Ren Fail. 2022. PMID: 35930288 Free PMC article. Review.
-
Store-operated calcium entry and diabetic complications.Exp Biol Med (Maywood). 2016 Feb;241(4):343-52. doi: 10.1177/1535370215609693. Epub 2015 Oct 14. Exp Biol Med (Maywood). 2016. PMID: 26468167 Free PMC article. Review.
-
Transcriptome-based analysis of kidney gene expression changes associated with diabetes in OVE26 mice, in the presence and absence of losartan treatment.PLoS One. 2014 May 14;9(5):e96987. doi: 10.1371/journal.pone.0096987. eCollection 2014. PLoS One. 2014. PMID: 24827579 Free PMC article.
References
-
- De Zeeuw D, Remuzzi G, Parving H, Keane W, Zhang Z, Shahinfar S, Snapinn S, Cooper M, Mitch W, Brenner B: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65: 2309– 2320 - PubMed
-
- Drummond K, Mauer M; International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes. II. Early renal structural changes in type 1 diabetes. Diabetes 2002; 51: 1580– 1587 - PubMed
-
- Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 1999; 42: 1341– 1344 - PubMed
-
- Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005; 54: 1626– 1634 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

