Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification
- PMID: 19202066
- PMCID: PMC2651314
- DOI: 10.1073/pnas.0813210106
Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification
Abstract
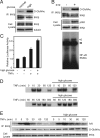
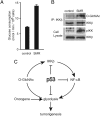
The IkappaB kinase (IKK)-NF-kappaB pathway plays a critical role in oncogenesis. Recently, we have shown that p53 regulates glucose metabolism through the IKK-NF-kappaB pathway and that, in the absence of p53, the positive feedback loop between IKK-NF-kappaB and glycolysis has an integral role in oncogene-induced cell transformation. Here, we demonstrate that IKKbeta, a component of the IKK complex, was constitutively modified with O-linked beta-N-acetyl glucosamine (O-GlcNAc) in both p53-deficient mouse embryonic fibroblasts (MEFs) and transformed human fibroblasts. In p53-deficient cells, the O-GlcNAcylated IKKbeta and the activating phosphorylation of IKK were decreased by p65/NF-kappaB knockdown or glucose depletion. We also found that high glucose induced the O-GlcNAcylation of IKKbeta and sustained the TNFalpha-dependent IKKbeta activity. Moreover, the O-GlcNAcase inhibitor streptozotocin intensified O-GlcNAcylation and concomitant activating phosphorylation of IKKbeta. Mutational analysis revealed that O-GlcNAcylation of IKKbeta occurred at Ser 733 in the C-terminal domain, which was identified as an inactivating phosphorylation site, suggesting that IKKbeta O-GlcNAcylation regulates its catalytic activity. Taken together, we propose a novel mechanism for the enhancement of NF-kappaB activity by loss of p53, which evokes positive feedback regulation from enhanced glucose metabolism to IKK in oncogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
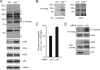


Similar articles
-
p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation.Nat Cell Biol. 2008 May;10(5):611-8. doi: 10.1038/ncb1724. Epub 2008 Apr 6. Nat Cell Biol. 2008. PMID: 18391940
-
Hyper-O-GlcNAcylation activates nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling through interplay with phosphorylation and acetylation.J Biol Chem. 2017 Jun 2;292(22):9150-9163. doi: 10.1074/jbc.M116.766568. Epub 2017 Apr 17. J Biol Chem. 2017. PMID: 28416608 Free PMC article.
-
A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity.J Biol Chem. 2005 Mar 18;280(11):10326-32. doi: 10.1074/jbc.M412643200. Epub 2004 Dec 20. J Biol Chem. 2005. PMID: 15611068
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Glycolysis links p53 function with NF-kappaB signaling: impact on cancer and aging process.J Cell Physiol. 2010 Jul;224(1):1-6. doi: 10.1002/jcp.22119. J Cell Physiol. 2010. PMID: 20301205 Review.
Cited by
-
Tools for investigating O-GlcNAc in signaling and other fundamental biological pathways.J Biol Chem. 2024 Feb;300(2):105615. doi: 10.1016/j.jbc.2023.105615. Epub 2023 Dec 29. J Biol Chem. 2024. PMID: 38159850 Free PMC article. Review.
-
Overview of Cancer Metabolism and Signaling Transduction.Int J Mol Sci. 2022 Dec 20;24(1):12. doi: 10.3390/ijms24010012. Int J Mol Sci. 2022. PMID: 36613455 Free PMC article. Review.
-
p53 and metabolism.Nat Rev Cancer. 2009 Oct;9(10):691-700. doi: 10.1038/nrc2715. Epub 2009 Sep 17. Nat Rev Cancer. 2009. PMID: 19759539 Review.
-
O-GlcNAcylation: A New Cancer Hallmark?Front Endocrinol (Lausanne). 2013 Aug 12;4:99. doi: 10.3389/fendo.2013.00099. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23964270 Free PMC article.
-
O-GlcNAcylation of RIPK1 rescues red blood cells from necroptosis.Front Immunol. 2023 Jun 9;14:1160490. doi: 10.3389/fimmu.2023.1160490. eCollection 2023. Front Immunol. 2023. PMID: 37359541 Free PMC article.
References
-
- Barnes PJ, Karin M. Nuclear factor-κB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. - PubMed
-
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. - PubMed
-
- Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 2005;26:318–325. - PubMed
-
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(Suppl):S81–S96. - PubMed
-
- Hu Y, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

