Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion
- PMID: 19196977
- PMCID: PMC2650357
- DOI: 10.1073/pnas.0813223106
Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion
Abstract
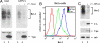
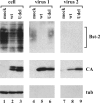
HIV-1 Vpu enhances the release of virions from infected cells. Recent work identified Bst-2/CD317/tetherin as a host factor whose inhibitory activity on viral release is counteracted by Vpu. A current working model proposes that Bst-2 inhibits virus release by tethering viral particles to the cell surface. Here, we analyzed endogenous Bst-2 with respect to its effect on virus release from HeLa cells, T cells, and macrophages. We noted significant cell type-dependent variation in Bst-2 expression. Vpu caused a reduction in Bst-2 expression in transfected HeLa cells and long-term infected macrophages. However, Vpu expression did not result in cell surface down-modulation of Bst-2 or a reduction in intracellular Bst-2 expression in CEMx174 or H9 cells, yet virus replication in these cells was Vpu-responsive. Surprisingly, Bst-2 was undetectable in cell-free virions that were recovered from the surface of HeLa cells by physical shearing, suggesting that a tethering model may not explain all of the functional properties of Bst-2. Taken together we conclude that enhancement of virus release by Vpu does not, at least in CEMx174 and H9 cells, require cell surface down-modulation or intracellular depletion of Bst-2, nor does it entail exclusion of Bst-2 from viral particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
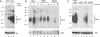

Similar articles
-
The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu.Retrovirology. 2009 Sep 8;6:80. doi: 10.1186/1742-4690-6-80. Retrovirology. 2009. PMID: 19737401 Free PMC article.
-
The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein.Cell Host Microbe. 2008 Apr 17;3(4):245-52. doi: 10.1016/j.chom.2008.03.001. Epub 2008 Mar 13. Cell Host Microbe. 2008. PMID: 18342597 Free PMC article.
-
Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism.J Virol. 2009 Aug;83(16):7931-47. doi: 10.1128/JVI.00242-09. Epub 2009 Jun 10. J Virol. 2009. PMID: 19515779 Free PMC article.
-
Sites of action of HIV-1 Vpu in BST-2/tetherin downregulation.Curr HIV Res. 2012 Jun;10(4):283-91. doi: 10.2174/157016212800792423. Curr HIV Res. 2012. PMID: 22524176 Review.
-
Interactions of viral protein U (Vpu) with cellular factors.Curr Top Microbiol Immunol. 2009;339:27-45. doi: 10.1007/978-3-642-02175-6_2. Curr Top Microbiol Immunol. 2009. PMID: 20012522 Review.
Cited by
-
Epigenetic Regulation of BST-2 Expression Levels and the Effect on HIV-1 Pathogenesis.Front Immunol. 2021 May 5;12:669241. doi: 10.3389/fimmu.2021.669241. eCollection 2021. Front Immunol. 2021. PMID: 34025670 Free PMC article.
-
BST2/tetherin inhibition of alphavirus exit.Viruses. 2015 Apr 22;7(4):2147-67. doi: 10.3390/v7042147. Viruses. 2015. PMID: 25912717 Free PMC article.
-
The HIV-1 Vpu viroporin inhibitor BIT225 does not affect Vpu-mediated tetherin antagonism.PLoS One. 2011;6(11):e27660. doi: 10.1371/journal.pone.0027660. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110710 Free PMC article.
-
Genetic characterization of natural variants of Vpu from HIV-1 infected individuals from Northern India and their impact on virus release and cell death.PLoS One. 2013;8(3):e59283. doi: 10.1371/journal.pone.0059283. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555649 Free PMC article.
-
Vpu and BST2: Still Not There Yet?Front Microbiol. 2012 Apr 9;3:131. doi: 10.3389/fmicb.2012.00131. eCollection 2012. Front Microbiol. 2012. PMID: 22509177 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

