The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits
- PMID: 19193898
- PMCID: PMC2746438
- DOI: 10.1523/JNEUROSCI.3937-08.2009
The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits
Abstract
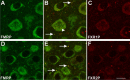
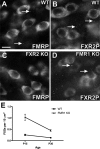
The loss of Fragile X mental retardation protein (FMRP) causes Fragile X syndrome, the most common inherited mental retardation and single gene cause of autism. Although postsynaptic functions for FMRP are well established, potential roles at the presynaptic apparatus remain largely unexplored. Here, we characterize the expression of FMRP and its homologs, FXR1P and FXR2P, in the developing, mature and regenerating rodent nervous system, with a focus on presynaptic expression. As expected, FMRP is expressed in the somatodendritic domain in virtually all neurons. However, FMRP is also localized in discrete granules (Fragile X granules; FXGs) in a subset of brain regions including frontal cortex, hippocampal area CA3 and olfactory bulb glomeruli. Immunoelectron microscopy shows that FMRP is localized at presynaptic terminals and in axons within these FXG-rich regions. With the exception of the olfactory bulb, FXGs are prominent only in the developing brain. Experiments in regenerating olfactory circuits indicate that peak FXG expression occurs 2-4 weeks after neurogenesis, a period that correlates with synapse formation and refinement. Virtually all FXGs contain FXR2P, while region-selective subsets harbor FMRP and/or FXR1P. Genetic studies show that FXR2P is essential for FXG expression, while FMRP regulates FXG number and developmental profile. These findings suggest that Fragile X proteins play a distinct, presynaptic role during discrete developmental epochs in defined circuits of the mammalian CNS. We propose that the neurological defects in Fragile X syndrome, including the autistic features, could be due in part to the loss of FMRP function in presynaptic compartments.
Figures


Similar articles
-
Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions.J Comp Neurol. 2012 Nov 1;520(16):3687-706. doi: 10.1002/cne.23123. J Comp Neurol. 2012. PMID: 22522693 Free PMC article.
-
Fragile X granules are a family of axonal ribonucleoprotein particles with circuit-dependent protein composition and mRNA cargos.J Comp Neurol. 2018 Jan 1;526(1):96-108. doi: 10.1002/cne.24321. Epub 2017 Sep 20. J Comp Neurol. 2018. PMID: 28884477 Free PMC article.
-
Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains.Hum Mol Genet. 2017 Jan 1;26(1):192-209. doi: 10.1093/hmg/ddw381. Hum Mol Genet. 2017. PMID: 28082376 Free PMC article.
-
The developmental roles of FMRP.Biochem Soc Trans. 2010 Apr;38(2):507-10. doi: 10.1042/BST0380507. Biochem Soc Trans. 2010. PMID: 20298211 Review.
-
Axonal and presynaptic FMRP: Localization, signal, and functional implications.Hear Res. 2023 Mar 15;430:108720. doi: 10.1016/j.heares.2023.108720. Epub 2023 Feb 11. Hear Res. 2023. PMID: 36809742 Free PMC article. Review.
Cited by
-
Cytoplasmic RNA-binding proteins and the control of complex brain function.Cold Spring Harb Perspect Biol. 2012 Aug 1;4(8):a012344. doi: 10.1101/cshperspect.a012344. Cold Spring Harb Perspect Biol. 2012. PMID: 22723494 Free PMC article. Review.
-
Regulation of neuronal gene expression by local axonal translation.Curr Genet Med Rep. 2016 Mar;4(1):16-25. doi: 10.1007/s40142-016-0085-2. Epub 2016 Feb 6. Curr Genet Med Rep. 2016. PMID: 27722035 Free PMC article.
-
Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P.Dis Model Mech. 2010 Jul-Aug;3(7-8):471-85. doi: 10.1242/dmm.004598. Epub 2010 May 4. Dis Model Mech. 2010. PMID: 20442204 Free PMC article.
-
Fragile X syndrome and associated disorders: Clinical aspects and pathology.Neurobiol Dis. 2020 Mar;136:104740. doi: 10.1016/j.nbd.2020.104740. Epub 2020 Jan 10. Neurobiol Dis. 2020. PMID: 31927143 Free PMC article. Review.
-
Molecular determinants of the axonal mRNA transcriptome.Dev Neurobiol. 2014 Mar;74(3):218-32. doi: 10.1002/dneu.22123. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23959706 Free PMC article. Review.
References
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–293. - PubMed
-
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
-
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. - PubMed
-
- Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 2000;258:162–170. - PubMed
-
- Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol. 1974;158:55–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC002167-10/DC/NIDCD NIH HHS/United States
- P20 RR015578-030002/RR/NCRR NIH HHS/United States
- DC002167/DC/NIDCD NIH HHS/United States
- R01 DC002167/DC/NIDCD NIH HHS/United States
- HD052083/HD/NICHD NIH HHS/United States
- P20 RR015578/RR/NCRR NIH HHS/United States
- R01 DC002167-13/DC/NIDCD NIH HHS/United States
- DA021501/DA/NIDA NIH HHS/United States
- P20 RR015578-03S10002/RR/NCRR NIH HHS/United States
- P20 RR015578-050002/RR/NCRR NIH HHS/United States
- R01 DC002167-12/DC/NIDCD NIH HHS/United States
- R01 HD052083-03/HD/NICHD NIH HHS/United States
- R01 HD052083-01/HD/NICHD NIH HHS/United States
- F32 DA021501-02/DA/NIDA NIH HHS/United States
- P20 RR015578-010002/RR/NCRR NIH HHS/United States
- P01 NS039321-05/NS/NINDS NIH HHS/United States
- P20 RR015578-01S10002/RR/NCRR NIH HHS/United States
- T32 AG020498-02/AG/NIA NIH HHS/United States
- P01 NS039321-02/NS/NINDS NIH HHS/United States
- T32 AG020498-01A1/AG/NIA NIH HHS/United States
- P01 NS039321-04/NS/NINDS NIH HHS/United States
- P20 RR015578-060002/RR/NCRR NIH HHS/United States
- P01 NS039321-01/NS/NINDS NIH HHS/United States
- T32 AG020498-03/AG/NIA NIH HHS/United States
- P01 NS039321/NS/NINDS NIH HHS/United States
- P20 RR015578-040002/RR/NCRR NIH HHS/United States
- AG02049/AG/NIA NIH HHS/United States
- R01 DC002167-14/DC/NIDCD NIH HHS/United States
- F32 DA021501/DA/NIDA NIH HHS/United States
- P01 NS039321-03/NS/NINDS NIH HHS/United States
- R01 HD052083/HD/NICHD NIH HHS/United States
- P20 RR015578-020002/RR/NCRR NIH HHS/United States
- R01 HD052083-02/HD/NICHD NIH HHS/United States
- R01 DC002167-11/DC/NIDCD NIH HHS/United States
- F32 DA021501-01A1/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
