Structural basis for recognition of diubiquitins by NEMO
- PMID: 19185524
- PMCID: PMC2749619
- DOI: 10.1016/j.molcel.2009.01.012
Structural basis for recognition of diubiquitins by NEMO
Abstract
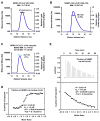
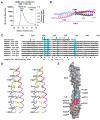
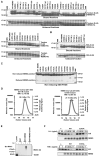
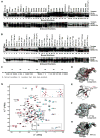
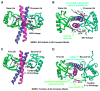
NEMO is the regulatory subunit of the IkappaB kinase (IKK) in NF-kappaB activation, and its CC2-LZ region interacts with Lys63 (K63)-linked polyubiquitin to recruit IKK to receptor signaling complexes. In vitro, CC2-LZ also interacts with tandem diubiquitin. Here we report the crystal structure of CC2-LZ with two dimeric coiled coils representing CC2 and LZ, respectively. Surprisingly, mutagenesis and nuclear magnetic resonance experiments reveal that the binding sites for diubiquitins at LZ are composites of both chains and that each ubiquitin in diubiquitins interacts with symmetrical NEMO asymmetrically. For tandem diubiquitin, the first ubiquitin uses the conserved hydrophobic patch and the C-terminal tail, while the second ubiquitin uses an adjacent surface patch. For K63-linked diubiquitin, the proximal ubiquitin uses its conserved hydrophobic patch, while the distal ubiquitin mostly employs the C-terminal arm including the K63 linkage residue. These studies uncover the energetics and geometry for mutual recognition of NEMO and diubiquitins.
Figures
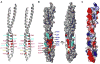

Similar articles
-
Direct inhibition of NF-κB activation by peptide targeting the NOA ubiquitin binding domain of NEMO.Biochem Pharmacol. 2011 Nov 1;82(9):1163-74. doi: 10.1016/j.bcp.2011.07.083. Epub 2011 Jul 23. Biochem Pharmacol. 2011. PMID: 21803029
-
Mechanism underlying IκB kinase activation mediated by the linear ubiquitin chain assembly complex.Mol Cell Biol. 2014 Apr;34(7):1322-35. doi: 10.1128/MCB.01538-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469399 Free PMC article.
-
DARPin-assisted crystallography of the CC2-LZ domain of NEMO reveals a coupling between dimerization and ubiquitin binding.J Mol Biol. 2010 Jan 8;395(1):89-104. doi: 10.1016/j.jmb.2009.10.018. Epub 2009 Oct 23. J Mol Biol. 2010. PMID: 19854204
-
Protein-protein interactions involving IKKgamma (NEMO) that promote the activation of NF-kappaB.J Cell Physiol. 2010 Jun;223(3):558-61. doi: 10.1002/jcp.22105. J Cell Physiol. 2010. PMID: 20301198 Review.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1.PLoS One. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. Epub 2012 Jul 26. PLoS One. 2012. PMID: 22848449 Free PMC article.
-
Structural insights into the assembly of large oligomeric signalosomes in the Toll-like receptor-interleukin-1 receptor superfamily.Sci Signal. 2012 May 29;5(226):re3. doi: 10.1126/scisignal.2003124. Sci Signal. 2012. PMID: 22649099 Free PMC article. Review.
-
Molecular basis of NF-κB signaling.Annu Rev Biophys. 2013;42:443-68. doi: 10.1146/annurev-biophys-083012-130338. Epub 2013 Mar 11. Annu Rev Biophys. 2013. PMID: 23495970 Free PMC article. Review.
-
Linear ubiquitination signals in adaptive immune responses.Immunol Rev. 2015 Jul;266(1):222-36. doi: 10.1111/imr.12300. Immunol Rev. 2015. PMID: 26085218 Free PMC article. Review.
-
NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain.EMBO J. 2009 Oct 7;28(19):2885-95. doi: 10.1038/emboj.2009.241. Epub 2009 Sep 17. EMBO J. 2009. PMID: 19763089 Free PMC article.
References
-
- Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israel A, Veron M. NEMO trimerizes through its coiled-coil C-terminal domain. J Biol Chem. 2002;277:17464–17475. - PubMed
-
- Cook WJ, Jeffrey LC, Kasperek E, Pickart CM. Structure of tetraubiquitin shows how multiubiquitin chains can be formed. J Mol Biol. 1994;236:601–609. - PubMed
-
- Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. - PubMed
-
- Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

