Dendritic cell homeostasis
- PMID: 19176316
- PMCID: PMC2668851
- DOI: 10.1182/blood-2008-12-180646
Dendritic cell homeostasis
Abstract
Dendritic cells (DCs) are a heterogeneous fraction of rare hematopoietic cells that coevolved with the formation of the adaptive immune system. DCs efficiently process and present antigen, move from sites of antigen uptake to sites of cellular interactions, and are critical in the initiation of immune responses as well as in the maintenance of self-tolerance. DCs are distributed throughout the body and are enriched in lymphoid organs and environmental contact sites. Steady-state DC half-lives account for days to up to a few weeks, and they need to be replaced via proliferating hematopoietic progenitors, monocytes, or tissue resident cells. In this review, we integrate recent knowledge on DC progenitors, cytokines, and transcription factor usage to an emerging concept of in vivo DC homeostasis in steady-state and inflammatory conditions. We furthermore highlight how knowledge of these maintenance mechanisms might impact on understanding of DC malignancies as well as posttransplant immune reactions and their respective therapies.
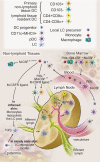
Figures

Similar articles
-
Complexity of dendritic cell subsets and their function in the host immune system.Immunology. 2011 Aug;133(4):409-19. doi: 10.1111/j.1365-2567.2011.03457.x. Epub 2011 Jun 1. Immunology. 2011. PMID: 21627652 Free PMC article. Review.
-
In Vitro Generation of Human XCR1(+) Dendritic Cells from CD34(+) Hematopoietic Progenitors.Methods Mol Biol. 2016;1423:19-37. doi: 10.1007/978-1-4939-3606-9_2. Methods Mol Biol. 2016. PMID: 27142006
-
The development and function of dendritic cell populations and their regulation by miRNAs.Protein Cell. 2017 Jul;8(7):501-513. doi: 10.1007/s13238-017-0398-2. Epub 2017 Mar 31. Protein Cell. 2017. PMID: 28364278 Free PMC article. Review.
-
Dendritic cell homeostasis is maintained by nonhematopoietic and T-cell-produced Flt3-ligand in steady state and during immune responses.Eur J Immunol. 2013 Jun;43(6):1651-8. doi: 10.1002/eji.201243163. Epub 2013 Apr 27. Eur J Immunol. 2013. PMID: 23519969
-
Molecular control of steady-state dendritic cell maturation and immune homeostasis.Annu Rev Immunol. 2013;31:743-91. doi: 10.1146/annurev-immunol-020711-074929. Epub 2013 Jan 17. Annu Rev Immunol. 2013. PMID: 23330953 Free PMC article. Review.
Cited by
-
C/EBPα is required for development of dendritic cell progenitors.Blood. 2013 May 16;121(20):4073-81. doi: 10.1182/blood-2012-10-463448. Epub 2013 Apr 1. Blood. 2013. PMID: 23547051 Free PMC article.
-
Functional Ambivalence of Dendritic Cells: Tolerogenicity and Immunogenicity.Int J Mol Sci. 2021 Apr 23;22(9):4430. doi: 10.3390/ijms22094430. Int J Mol Sci. 2021. PMID: 33922658 Free PMC article. Review.
-
The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice.J Clin Invest. 2012 May;122(5):1615-27. doi: 10.1172/JCI60644. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505458 Free PMC article.
-
The colony-stimulating factors and cancer.Nat Rev Cancer. 2010 Jun;10(6):425-34. doi: 10.1038/nrc2843. Nat Rev Cancer. 2010. PMID: 20495576 Free PMC article. Review.
-
Polyelectrolyte Coating of Ferumoxytol Differentially Impacts the Labeling of Inflammatory and Steady-State Dendritic Cell Subtypes.Biomedicines. 2022 Dec 5;10(12):3137. doi: 10.3390/biomedicines10123137. Biomedicines. 2022. PMID: 36551893 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. - PubMed
-
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

