Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+ -induced glucagon exocytosis in pancreas
- PMID: 19171650
- PMCID: PMC2674989
- DOI: 10.1113/jphysiol.2008.168005
Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+ -induced glucagon exocytosis in pancreas
Abstract
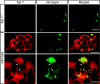
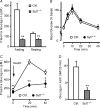
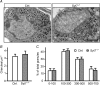
Hormones such as glucagon are secreted by Ca(2+)-induced exocytosis of large dense-core vesicles, but the mechanisms involved have only been partially elucidated. Studies of pancreatic beta-cells secreting insulin revealed that synaptotagmin-7 alone is not sufficient to mediate Ca(2+)-dependent insulin granule exocytosis, and studies of chromaffin cells secreting neuropeptides and catecholamines showed that synaptotagmin-1 and -7 collaborate as Ca(2+) sensors for exocytosis, and that both are equally involved. As no other peptide secretion was analysed, it remains unclear whether synaptotagmins generally act as Ca(2+) sensors in large dense-core vesicle exocytosis in endocrine cells, and if so, whether synaptotagmin-7 always functions with a partner in that role. In particular, far less is known about the mechanisms underlying Ca(2+)-triggered glucagon release from alpha-cells than insulin secretion from beta-cells, even though insulin and glucagon together regulate blood glucose levels. To address these issues, we analysed the role of synaptotagmins in Ca(2+)-triggered glucagon exocytosis. Surprisingly, we find that deletion of a single synaptotagmin isoform, synaptotagmin-7, nearly abolished Ca(2+)-triggered glucagon secretion. Moreover, single-cell capacitance measurements confirmed that pancreatic alpha-cells lacking synaptotagmin-7 exhibited little Ca(2+)-induced exocytosis, whereas all other physiological and morphological parameters of the alpha-cells were normal. Our data thus identify synaptotagmin-7 as a principal Ca(2+) sensor for glucagon secretion, and support the notion that synaptotagmins perform a universal but selective function as individually acting Ca(2+) sensors in neurotransmitter, neuropeptide, and hormone secretion.
Figures
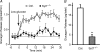

Similar articles
-
Neuronal calcium sensor synaptotagmin-9 is not involved in the regulation of glucose homeostasis or insulin secretion.PLoS One. 2010 Nov 9;5(11):e15414. doi: 10.1371/journal.pone.0015414. PLoS One. 2010. PMID: 21085706 Free PMC article.
-
Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells.Proc Natl Acad Sci U S A. 2008 Mar 11;105(10):3998-4003. doi: 10.1073/pnas.0712373105. Epub 2008 Feb 28. Proc Natl Acad Sci U S A. 2008. PMID: 18308932 Free PMC article.
-
Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans.J Physiol. 2004 May 1;556(Pt 3):711-26. doi: 10.1113/jphysiol.2003.059675. Epub 2004 Feb 13. J Physiol. 2004. PMID: 14966302 Free PMC article.
-
Synaptotagmins bind calcium to release insulin.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1279-86. doi: 10.1152/ajpendo.90568.2008. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713958 Review.
-
Synaptotagmin IV acts as a multi-functional regulator of Ca2+-dependent exocytosis.Neurochem Res. 2011 Jul;36(7):1222-7. doi: 10.1007/s11064-010-0352-7. Epub 2010 Dec 10. Neurochem Res. 2011. PMID: 21153436 Review.
Cited by
-
Role of vesicle-associated membrane protein 2 in exocytosis of glucagon-like peptide-1 from the murine intestinal L cell.Diabetologia. 2014 Apr;57(4):809-18. doi: 10.1007/s00125-013-3143-2. Epub 2013 Dec 20. Diabetologia. 2014. PMID: 24356748
-
5-IP7 is a GPCR messenger mediating neural control of synaptotagmin-dependent insulin exocytosis and glucose homeostasis.Nat Metab. 2021 Oct;3(10):1400-1414. doi: 10.1038/s42255-021-00468-7. Epub 2021 Oct 18. Nat Metab. 2021. PMID: 34663975
-
Synaptotagmin-7 Functions to Replenish Insulin Granules for Exocytosis in Human Islet β-Cells.Diabetes. 2016 Jul;65(7):1962-76. doi: 10.2337/db15-1436. Epub 2016 Apr 26. Diabetes. 2016. PMID: 27207520 Free PMC article.
-
Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation.J Cancer. 2018 Jun 12;9(13):2349-2356. doi: 10.7150/jca.25098. eCollection 2018. J Cancer. 2018. PMID: 30026831 Free PMC article.
-
A manual collection of Syt, Esyt, Rph3a, Rph3al, Doc2, and Dblc2 genes from 46 metazoan genomes--an open access resource for neuroscience and evolutionary biology.BMC Genomics. 2010 Jan 15;11:37. doi: 10.1186/1471-2164-11-37. BMC Genomics. 2010. PMID: 20078875 Free PMC article.
References
-
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet β-cells. J Biol Chem. 2000;275:36079–36085. - PubMed
-
- Gauthier BR, Duhamel DL, Iezzi M, Theander S, Saltel F, Fukuda M, Wehrle-Haller B, Wollheim CB. Synaptotagmin VII splice variants α, β, and δ are expressed in pancreatic β-cells and regulate insulin exocytosis. FASEB J. 2008;22:194–206. - PubMed
-
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. - PubMed
-
- Gerber SH, Südhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51(Suppl 1):S3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

