Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen
- PMID: 19168629
- PMCID: PMC2635812
- DOI: 10.1073/pnas.0812247106
Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen
Abstract
Dendritic cells (DCs) are strategically positioned to take up antigens and initiate adaptive immunity. One DC subset expresses CD8alphaalpha in mice and is specialized to capture dying cells and process antigens for MHC class I "cross-presentation." Because CD8(+) DCs also express DEC205/CD205, which is localized to splenic T cell regions, it is thought that CD8(+) DCs also are restricted to T zones. Here, we used a new antibody to Langerin/CD207, which colabels isolated CD8(+) CD205(+) DCs, to immunolabel spleen sections. The mAb labeled discrete cells with high levels of CD11c and CD8. Surprisingly most CD207(+) profiles were in marginal zones surrounding splenic white pulp nodules, and only smaller numbers were in T cell areas, where CD205 colabeling was noted. Despite a marginal zone location, CD207(+) DCs lacked identifying molecules for 3 different types of macrophages, localized in proximity and, in contrast to macrophages, marginal zone DCs were poor scavengers of soluble and particulate substrates. After stimulation with microbial agonists, Langerin expression disappeared from the marginal zone at 6-12 h, but was greatly expanded in the T cell areas, and by 24-48 h, Langerin expression disappeared. Therefore, anti-Langerin antibodies localize a majority of CD8(+) DCs to non-T cell regions of mouse spleen, where they are distinct from adjacent macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
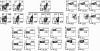
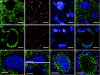
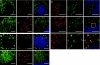

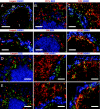

Similar articles
-
Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A.Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2384-9. doi: 10.1073/pnas.1019547108. Epub 2011 Jan 24. Proc Natl Acad Sci U S A. 2011. PMID: 21262813 Free PMC article.
-
Batf3-independent langerin- CX3CR1- CD8α+ splenic DCs represent a precursor for classical cross-presenting CD8α+ DCs.J Leukoc Biol. 2014 Dec;96(6):1001-10. doi: 10.1189/jlb.1A0314-130R. Epub 2014 Aug 28. J Leukoc Biol. 2014. PMID: 25170118
-
Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo.J Immunol. 2008 Mar 15;180(6):3647-50. doi: 10.4049/jimmunol.180.6.3647. J Immunol. 2008. PMID: 18322168
-
Langerin+CD8+ Dendritic Cells in the Splenic Marginal Zone: Not So Marginal After All.Front Immunol. 2019 Apr 12;10:741. doi: 10.3389/fimmu.2019.00741. eCollection 2019. Front Immunol. 2019. PMID: 31031751 Free PMC article. Review.
-
Langerin-expressing dendritic cells in gut-associated lymphoid tissues.Immunol Rev. 2010 Mar;234(1):233-46. doi: 10.1111/j.0105-2896.2009.00878.x. Immunol Rev. 2010. PMID: 20193022 Review.
Cited by
-
Host Langerin (CD207) is a receptor for Yersinia pestis phagocytosis and promotes dissemination.Immunol Cell Biol. 2015 Oct;93(9):815-24. doi: 10.1038/icb.2015.46. Epub 2015 Apr 1. Immunol Cell Biol. 2015. PMID: 25829141 Free PMC article.
-
Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages.Nat Immunol. 2012 Nov;13(11):1118-28. doi: 10.1038/ni.2419. Epub 2012 Sep 30. Nat Immunol. 2012. PMID: 23023392 Free PMC article.
-
A systems biology approach to the analysis of subset-specific responses to lipopolysaccharide in dendritic cells.PLoS One. 2014 Jun 20;9(6):e100613. doi: 10.1371/journal.pone.0100613. eCollection 2014. PLoS One. 2014. PMID: 24949855 Free PMC article.
-
A protective role of murine langerin⁺ cells in immune responses to cutaneous vaccination with microneedle patches.Sci Rep. 2014 Aug 18;4:6094. doi: 10.1038/srep06094. Sci Rep. 2014. PMID: 25130187 Free PMC article.
-
Development and function of chicken XCR1+ conventional dendritic cells.Front Immunol. 2023 Oct 25;14:1273661. doi: 10.3389/fimmu.2023.1273661. eCollection 2023. Front Immunol. 2023. PMID: 37954617 Free PMC article.
References
-
- Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. - PubMed
-
- Steinman RM. Dendritic cells in vivo: A key target for a new vaccine science. Immunity. 2008;29:319–324. - PubMed
-
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. - PubMed
-
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
-
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

