Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells
- PMID: 19167330
- PMCID: PMC2805151
- DOI: 10.1016/j.cell.2008.11.037
Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells
Abstract
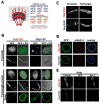
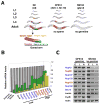
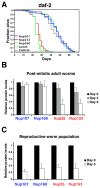
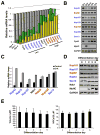
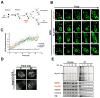
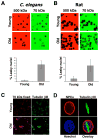
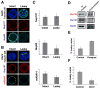
In dividing cells, nuclear pore complexes (NPCs) disassemble during mitosis and reassemble into the newly forming nuclei. However, the fate of nuclear pores in postmitotic cells is unknown. Here, we show that NPCs, unlike other nuclear structures, do not turn over in differentiated cells. While a subset of NPC components, like Nup153 and Nup50, are continuously exchanged, scaffold nucleoporins, like the Nup107/160 complex, are extremely long-lived and remain incorporated in the nuclear membrane during the entire cellular life span. Besides the lack of nucleoporin expression and NPC turnover, we discovered an age-related deterioration of NPCs, leading to an increase in nuclear permeability and the leaking of cytoplasmic proteins into the nucleus. Our finding that nuclear "leakiness" is dramatically accelerated during aging and that a subset of nucleoporins is oxidatively damaged in old cells suggests that the accumulation of damage at the NPC might be a crucial aging event.
Figures
Comment in
-
Old nuclei spring new leaks.Cell. 2009 Jan 23;136(2):211-2. doi: 10.1016/j.cell.2009.01.004. Cell. 2009. PMID: 19167324
Similar articles
-
Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex.Mol Biol Cell. 2003 May;14(5):1923-40. doi: 10.1091/mbc.e02-09-0620. Mol Biol Cell. 2003. PMID: 12802065 Free PMC article.
-
Nuclear pore complex maintenance and implications for age-related diseases.Trends Cell Biol. 2022 Mar;32(3):216-227. doi: 10.1016/j.tcb.2021.10.001. Epub 2021 Nov 12. Trends Cell Biol. 2022. PMID: 34782239 Review.
-
MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly.EMBO Rep. 2007 Feb;8(2):165-72. doi: 10.1038/sj.embor.7400889. Epub 2007 Jan 19. EMBO Rep. 2007. PMID: 17235358 Free PMC article.
-
The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly.J Cell Biol. 2009 May 4;185(3):459-73. doi: 10.1083/jcb.200810029. J Cell Biol. 2009. PMID: 19414608 Free PMC article.
-
Autophagy as a caretaker of nuclear integrity.FEBS Lett. 2023 Nov;597(22):2728-2738. doi: 10.1002/1873-3468.14719. Epub 2023 Aug 20. FEBS Lett. 2023. PMID: 37567863 Review.
Cited by
-
The pathophysiology of neurodegenerative disease: Disturbing the balance between phase separation and irreversible aggregation.Prog Mol Biol Transl Sci. 2020;174:187-223. doi: 10.1016/bs.pmbts.2020.04.021. Epub 2020 May 12. Prog Mol Biol Transl Sci. 2020. PMID: 32828466 Free PMC article. Review.
-
MstX and a putative potassium channel facilitate biofilm formation in Bacillus subtilis.PLoS One. 2013 May 30;8(5):e60993. doi: 10.1371/journal.pone.0060993. Print 2013. PLoS One. 2013. PMID: 23737939 Free PMC article.
-
The role of DNA damage response in amyotrophic lateral sclerosis.Essays Biochem. 2020 Oct 26;64(5):847-861. doi: 10.1042/EBC20200002. Essays Biochem. 2020. PMID: 33078197 Free PMC article. Review.
-
PhOTO zebrafish: a transgenic resource for in vivo lineage tracing during development and regeneration.PLoS One. 2012;7(3):e32888. doi: 10.1371/journal.pone.0032888. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431986 Free PMC article.
-
Aging and physiological barriers: mechanisms of barrier integrity changes and implications for age-related diseases.Mol Biol Rep. 2024 Aug 19;51(1):917. doi: 10.1007/s11033-024-09833-7. Mol Biol Rep. 2024. PMID: 39158744 Review.
References
-
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. - PubMed
-
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
-
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The Integral Membrane Nucleoporin pom121 Functionally Links Nuclear Pore Complex Assembly and Nuclear Envelope Formation. Mol Cell. 2005;17:83–92. - PubMed
-
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell. 1998;95:199–210. - PubMed
-
- Beanan MJ, Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992;116:755–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

