Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5
- PMID: 19166829
- PMCID: PMC2694185
- DOI: 10.1016/j.ydbio.2008.12.028
Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5
Abstract
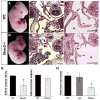
The pacemaker is composed of specialized cardiomyocytes located within the sinoatrial node (SAN), and is responsible for originating and regulating the heart beat. Recent advances towards understanding the SAN development have been made on the genetic control and gene interaction within this structure. Here we report that the Shox2 homeodomain transcription factor is restrictedly expressed in the sinus venosus region including the SAN and the sinus valves during embryonic heart development. Shox2 null mutation results in embryonic lethality due to cardiovascular defects, including an abnormal low heart beat rate (bradycardia) and severely hypoplastic SAN and sinus valves attributed to a significantly decreased level of cell proliferation. Genetically, the lack of Tbx3 and Hcn4 expression, along with ectopic activation of Nppa, Cx40, and Nkx2-5 in the Shox2(-/-) SAN region, indicates a failure in SAN differentiation. Furthermore, Shox2 overexpression in Xenopus embryos results in extensive repression of Nkx2-5 in the developing heart, leading to a reduced cardiac field and aberrant heart formation. Reporter gene expression assays provide additional evidence for the repression of Nkx2-5 promoter activity by Shox2. Taken together our results demonstrate that Shox2 plays an essential role in the SAN and pacemaker development by controlling a genetic cascade through the repression of Nkx2-5.
Figures



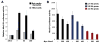

Similar articles
-
The role of Shox2 in SAN development and function.Pediatr Cardiol. 2012 Aug;33(6):882-9. doi: 10.1007/s00246-012-0179-x. Epub 2012 Feb 4. Pediatr Cardiol. 2012. PMID: 22307400
-
A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node.Development. 2015 Jul 15;142(14):2521-32. doi: 10.1242/dev.120220. Epub 2015 Jul 2. Development. 2015. PMID: 26138475 Free PMC article.
-
Phosphorylation of Shox2 is required for its function to control sinoatrial node formation.J Am Heart Assoc. 2014 May 20;3(3):e000796. doi: 10.1161/JAHA.114.000796. J Am Heart Assoc. 2014. PMID: 24847033 Free PMC article.
-
Shox2: The Role in Differentiation and Development of Cardiac Conduction System.Tohoku J Exp Med. 2018 Mar;244(3):177-186. doi: 10.1620/tjem.244.177. Tohoku J Exp Med. 2018. PMID: 29503396 Review.
-
Building the heart piece by piece: modularity of cis-elements regulating Nkx2-5 transcription.Development. 1999 Oct;126(19):4187-92. doi: 10.1242/dev.126.19.4187. Development. 1999. PMID: 10477287 Review.
Cited by
-
A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles.Cell Stem Cell. 2019 May 2;24(5):802-811.e5. doi: 10.1016/j.stem.2019.02.015. Epub 2019 Mar 14. Cell Stem Cell. 2019. PMID: 30880024 Free PMC article.
-
Atrial and Sinoatrial Node Development in the Zebrafish Heart.J Cardiovasc Dev Dis. 2021 Feb 9;8(2):15. doi: 10.3390/jcdd8020015. J Cardiovasc Dev Dis. 2021. PMID: 33572147 Free PMC article. Review.
-
Morpho-functional characterization of the systemic venous pole of the reptile heart.Sci Rep. 2017 Jul 27;7(1):6644. doi: 10.1038/s41598-017-06291-z. Sci Rep. 2017. PMID: 28751678 Free PMC article.
-
NODAL inhibition promotes differentiation of pacemaker-like cardiomyocytes from human induced pluripotent stem cells.Stem Cell Res. 2020 Dec;49:102043. doi: 10.1016/j.scr.2020.102043. Epub 2020 Oct 12. Stem Cell Res. 2020. PMID: 33128951 Free PMC article.
-
Transcription factor ISL1 is essential for pacemaker development and function.J Clin Invest. 2015 Aug 3;125(8):3256-68. doi: 10.1172/JCI68257. Epub 2015 Jul 20. J Clin Invest. 2015. PMID: 26193633 Free PMC article.
References
-
- Accili EA, Proenza C, Baruscotti M, Di Francesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. - PubMed
-
- Alappat S, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen YP. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol. 2005;277:102–113. - PubMed
-
- Belin V, Cusin V, Viot G, Girlich D, Toutain A, Moncla A, Vekemans M, Le Merrer M, Munnich A, Cormier-Daire V. SHOX mutations in dyschondrosteosis (Leri–Weill syndrome) Nat Genet. 1998;19:67–69. - PubMed
-
- Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson MC, Watson MS, Seidman JG, Seidman CE, Plowden J, Kugler JD. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. Clin Invest. 1999;104:1567–1573. - PMC - PubMed
-
- Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, Peña-Castillo L, Alleyne TM, Mnaimneh S, Botvinnik OB, Chan ET, Khalid F, Zhang W, Newburger D, Jaeger SA, Morris QD, Bulyk ML, Hughes TR. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133:1266–1276. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

