Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux
- PMID: 19166511
- PMCID: PMC3902824
- DOI: 10.1111/j.1471-4159.2009.05932.x
Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux
Abstract
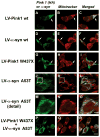
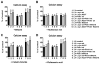
Parkinson's disease (PD) is characterized by accumulation of alpha-synuclein (alpha-syn) and degeneration of neuronal populations in cortical and subcortical regions. Mitochondrial dysfunction has been considered a potential unifying factor in the pathogenesis of the disease. Mutations in genes linked to familial forms of PD, including SNCA encoding alpha-syn and Pten-induced putative kinase 1 (PINK1), have been shown to disrupt mitochondrial activity. We investigated the mechanisms through which mutant Pink1 might disrupt mitochondrial function in neuronal cells with alpha-syn accumulation. For this purpose, a neuronal cell model of PD was infected with virally-delivered Pink1, and was analyzed for cell survival, mitochondrial activity and calcium flux. Mitochondrial morphology was analyzed by confocal and electron microscopy. These studies showed that mutant (W437X) but not wildtype Pink1 exacerbated the alterations in mitochondrial function promoted by mutant (A53T) alpha-syn. This effect was associated with increased intracellular calcium levels. Co-expression of both mutant Pink1 and alpha-syn led to alterations in mitochondrial structure and neurite outgrowth that were partially ameliorated by treatment with cyclosporine A, and completely restored by treatment with the mitochondrial calcium influx blocker Ruthenium Red, but not with other cellular calcium flux blockers. Our data suggest a role for mitochondrial calcium influx in the mechanisms of mitochondrial and neuronal dysfunction in PD. Moreover, these studies support an important function for Pink1 in regulating mitochondrial activity under stress conditions.
Figures

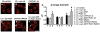


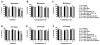
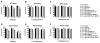
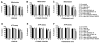
Similar articles
-
Impairment of mitochondria dynamics by human A53T α-synuclein and rescue by NAP (davunetide) in a cell model for Parkinson's disease.Exp Brain Res. 2017 Mar;235(3):731-742. doi: 10.1007/s00221-016-4836-9. Epub 2016 Nov 19. Exp Brain Res. 2017. PMID: 27866262 Free PMC article.
-
Alterations in α-synuclein and PINK1 expression reduce neurite length and induce mitochondrial fission and Golgi fragmentation in midbrain neurons.Neurosci Lett. 2020 Feb 16;720:134777. doi: 10.1016/j.neulet.2020.134777. Epub 2020 Jan 21. Neurosci Lett. 2020. PMID: 31978495
-
Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin.Nature. 2006 Jun 29;441(7097):1162-6. doi: 10.1038/nature04779. Epub 2006 May 3. Nature. 2006. PMID: 16672981
-
Function of α-synuclein and PINK1 in Lewy body dementia (Review).Int J Mol Med. 2015 Jan;35(1):3-9. doi: 10.3892/ijmm.2014.1980. Epub 2014 Oct 27. Int J Mol Med. 2015. PMID: 25355138 Review.
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
Cited by
-
Unconventional PINK1 localization to the outer membrane of depolarized mitochondria drives Parkin recruitment.J Cell Sci. 2015 Mar 1;128(5):964-78. doi: 10.1242/jcs.161000. Epub 2015 Jan 20. J Cell Sci. 2015. PMID: 25609704 Free PMC article.
-
Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration.Transl Neurodegener. 2022 Jan 25;11(1):3. doi: 10.1186/s40035-021-00278-7. Transl Neurodegener. 2022. PMID: 35078537 Free PMC article. Review.
-
Tickled PINK1: mitochondrial homeostasis and autophagy in recessive Parkinsonism.Biochim Biophys Acta. 2010 Jan;1802(1):20-8. doi: 10.1016/j.bbadis.2009.06.012. Epub 2009 Jul 9. Biochim Biophys Acta. 2010. PMID: 19595762 Free PMC article. Review.
-
Reappraisal of metabolic dysfunction in neurodegeneration: Focus on mitochondrial function and calcium signaling.Acta Neuropathol Commun. 2021 Jul 7;9(1):124. doi: 10.1186/s40478-021-01224-4. Acta Neuropathol Commun. 2021. PMID: 34233766 Free PMC article. Review.
-
Cellular and molecular pathophysiology in the progression of Parkinson's disease.Metab Brain Dis. 2021 Jun;36(5):815-827. doi: 10.1007/s11011-021-00689-5. Epub 2021 Feb 18. Metab Brain Dis. 2021. PMID: 33599945 Free PMC article. Review.
References
-
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
-
- Adamczyk A, Strosznajder JB. Alpha-synuclein potentiates Ca2+ influx through voltage-dependent Ca2+ channels. Neuroreport. 2006;17:1883–1886. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
-
- Bonifati V, Rohe CF, Breedveld GJ, et al. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. - PubMed
-
- Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

