Islet specific Wnt activation in human type II diabetes
- PMID: 19165345
- PMCID: PMC2628766
- DOI: 10.1155/2008/728763
Islet specific Wnt activation in human type II diabetes
Abstract
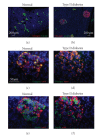
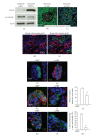
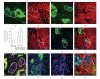
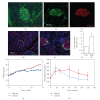
The Wnt pathway effector gene TCF7L2 has been linked to type II diabetes, making it important to study the role of Wnt signaling in diabetes pathogenesis. We examined the expression of multiple Wnt pathway components in pancreases from normal individuals and type II diabetic individuals. Multiple members of the Wnt signaling pathway, including TCF7L2, Wnt2b, beta-catenin, pGSK3beta, TCF3, cyclinD1, and c-myc, were undetectable or expressed at low levels in islets from nondiabetic individuals, but were also upregulated specifically in islets of type II diabetic patients. Culture of pancreatic tissue and islet isolation led to Wnt activation that was reversed by the Wnt antagonist sFRP, demonstrating that Wnt activation in that setting was due to soluble Wnt factors. These data support a model in which the Wnt pathway plays a dynamic role in the pathogenesis of type II diabetes and suggest manipulation of Wnt signaling as a new approach to beta-cell-directed diabetes therapy.
Figures


Similar articles
-
Regulation of wingless-type MMTV integration site family (WNT) signalling in pancreatic islets from wild-type and obese mice.Diabetologia. 2010 Jan;53(1):123-7. doi: 10.1007/s00125-009-1578-2. Epub 2009 Nov 8. Diabetologia. 2010. PMID: 19898815
-
The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus.Mol Endocrinol. 2008 Nov;22(11):2383-92. doi: 10.1210/me.2008-0135. Epub 2008 Jul 3. Mol Endocrinol. 2008. PMID: 18599616 Review.
-
Wnt signaling in pancreatic islets.Adv Exp Med Biol. 2010;654:391-419. doi: 10.1007/978-90-481-3271-3_17. Adv Exp Med Biol. 2010. PMID: 20217507 Review.
-
TCF7L2 and type 2 diabetes--we WNT to know.Diabetologia. 2007 Jan;50(1):5-7. doi: 10.1007/s00125-006-0521-z. Epub 2006 Nov 11. Diabetologia. 2007. PMID: 17102948 No abstract available.
-
Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway.Cell Death Dis. 2015 May 7;6(5):e1746. doi: 10.1038/cddis.2015.107. Cell Death Dis. 2015. PMID: 25950476 Free PMC article.
Cited by
-
Interaction of Wnt pathway related variants with type 2 diabetes in a Chinese Han population.PeerJ. 2015 Oct 20;3:e1304. doi: 10.7717/peerj.1304. eCollection 2015. PeerJ. 2015. PMID: 26509107 Free PMC article.
-
Approaches to Inducing β-Cell Regeneration.Biomedicines. 2022 Feb 28;10(3):571. doi: 10.3390/biomedicines10030571. Biomedicines. 2022. PMID: 35327373 Free PMC article. Review.
-
MYC: there is more to it than cancer.Front Cell Dev Biol. 2024 Mar 6;12:1342872. doi: 10.3389/fcell.2024.1342872. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38510176 Free PMC article. Review.
-
The effect of a preparation of minerals, vitamins and trace elements on the cardiac gene expression pattern in male diabetic rats.Cardiovasc Diabetol. 2015 Jun 28;14:85. doi: 10.1186/s12933-015-0248-6. Cardiovasc Diabetol. 2015. PMID: 26126619 Free PMC article.
-
The complex role of Wnt ligands in type 2 diabetes mellitus and related complications.J Cell Mol Med. 2021 Jul;25(14):6479-6495. doi: 10.1111/jcmm.16663. Epub 2021 May 27. J Cell Mol Med. 2021. PMID: 34042263 Free PMC article. Review.
References
-
- Porte D, Jr., Kahn SE. The key role of islet dysfunction in type II diabetes mellitus. Clinical and Investigative Medicine. 1995;18(4):247–254. - PubMed
-
- Poitout V, Briaud I, Kelpe C, Hagman D. Gluco-lipotoxicity of the pancreatic beta cell. Annales d'Endocrinologie. 2004;65(1):37–41. - PubMed
-
- Gunton JE, Kulkarni RN, Yim S, et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. - PubMed
-
- Robertson AP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. The Journal of Biological Chemistry. 2004;279(41):42351–42354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

