Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy
- PMID: 19164591
- PMCID: PMC2633563
- DOI: 10.1073/pnas.0805453106
Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy
Abstract
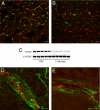
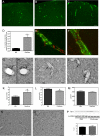
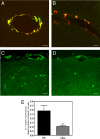
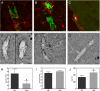
Cerebral amyloid angiopathy (CAA), the deposition of beta-amyloid (Abeta) peptides in leptomeningeal and cortical blood vessels, affects the majority of patients with Alzheimer's disease (AD). Evidence suggests that vascular amyloid deposits may result from impaired clearance of neuronal Abeta along perivascular spaces. We investigated the role of perivascular macrophages in regulating CAA severity in the TgCRND8 mouse model of AD. Depletion of perivascular macrophages significantly increased the number of thioflavin S-positive cortical blood vessels. ELISA confirmed that this increase was underscored by elevations in total vascular Abeta(42) levels. Conversely, stimulation of perivascular macrophage turnover reduced cerebral CAA load, an effect that was not mediated through clearance by microglia or astrocytes. These results highlight a function for the physiological role of perivascular macrophages in the regulation of CAA and suggest that selective targeting of perivascular macrophage activation might constitute a therapeutic strategy to clear vascular amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cerebral amyloid angiopathy aggravates perivascular clearance impairment in an Alzheimer's disease mouse model.Acta Neuropathol Commun. 2020 Nov 5;8(1):181. doi: 10.1186/s40478-020-01042-0. Acta Neuropathol Commun. 2020. PMID: 33153499 Free PMC article.
-
Capillary and arterial cerebral amyloid angiopathy in Alzheimer's disease: defining the perivascular route for the elimination of amyloid beta from the human brain.Neuropathol Appl Neurobiol. 2003 Apr;29(2):106-17. doi: 10.1046/j.1365-2990.2003.00424.x. Neuropathol Appl Neurobiol. 2003. PMID: 12662319
-
Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer's disease.Mol Med. 2003 Mar-Apr;9(3-4):112-22. Mol Med. 2003. PMID: 12865947 Free PMC article.
-
Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways.Nat Rev Neurol. 2020 Jan;16(1):30-42. doi: 10.1038/s41582-019-0281-2. Epub 2019 Dec 11. Nat Rev Neurol. 2020. PMID: 31827267 Free PMC article. Review.
-
Cerebral amyloid angiopathy.Prog Mol Biol Transl Sci. 2012;107:41-78. doi: 10.1016/B978-0-12-385883-2.00006-0. Prog Mol Biol Transl Sci. 2012. PMID: 22482447 Review.
Cited by
-
Deciphering the heterogeneity of the Lyve1+ perivascular macrophages in the mouse brain.Nat Commun. 2022 Nov 30;13(1):7366. doi: 10.1038/s41467-022-35166-9. Nat Commun. 2022. PMID: 36450771 Free PMC article.
-
The niche matters: origin, function and fate of CNS-associated macrophages during health and disease.Acta Neuropathol. 2024 Feb 12;147(1):37. doi: 10.1007/s00401-023-02676-9. Acta Neuropathol. 2024. PMID: 38347231 Free PMC article. Review.
-
The BET-Bromodomain Inhibitor JQ1 Reduces Inflammation and Tau Phosphorylation at Ser396 in the Brain of the 3xTg Model of Alzheimer's Disease.Curr Alzheimer Res. 2016;13(9):985-95. doi: 10.2174/1567205013666160427101832. Curr Alzheimer Res. 2016. PMID: 27117003 Free PMC article.
-
High-definition characterization of cerebral β-amyloid angiopathy in Alzheimer's disease.Hum Pathol. 2010 Nov;41(11):1601-8. doi: 10.1016/j.humpath.2010.04.011. Epub 2010 Aug 4. Hum Pathol. 2010. PMID: 20688356 Free PMC article.
-
The Vascular-Immune Hypothesis of Alzheimer's Disease.Biomedicines. 2023 Jan 30;11(2):408. doi: 10.3390/biomedicines11020408. Biomedicines. 2023. PMID: 36830944 Free PMC article. Review.
References
-
- Attems J, Lauda F, Jellinger KA. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J Neurol. 2008;255:70–76. - PubMed
-
- Shin HK, et al. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. - PubMed
-
- Perlmutter LS. Microvascular pathology and vascular basement membrane components in Alzheimer's disease. Mol Neurobiol. 1994;9:33–40. - PubMed
-
- Tian J, et al. Relationships in Alzheimer's disease between the extent of Abeta deposition in cerebral blood vessel walls, as cerebral amyloid angiopathy, and the amount of cerebrovascular smooth muscle cells and collagen. Neuropathol Appl Neurobiol. 2006;32:332–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

