1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4-2 prostate cancer cells
- PMID: 19164469
- PMCID: PMC2671895
- DOI: 10.1210/en.2008-1395
1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4-2 prostate cancer cells
Abstract
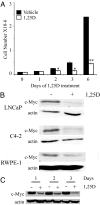
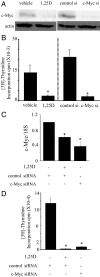
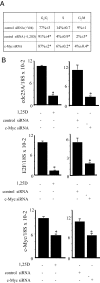
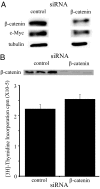
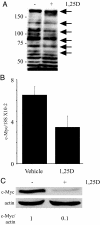
There is an inverse correlation between exposure to sunlight (the major source of vitamin D) and the risk for prostate cancer, the most common noncutaneous cancer and second most common cause of death from cancer in American men. The active metabolite of vitamin D, 1alpha,25-dihydroxyvitamin D(3) [1,25(OH)(2)D(3)] acting through the vitamin D receptor decreases prostate cancer cell growth and invasiveness. The precise mechanisms by which 1,25(OH)(2)D(3) inhibits growth in prostate cancer have not been fully elucidated. Treatment with 1,25(OH)(2)D(3) causes an accumulation in the G(0)/G(1) phase of the cell cycle in several prostate cancer cell lines. One potential target known to regulate the G(0)/G(1) to S phase transition is c-Myc, a transcription factor whose overexpression is associated with a number of cancers including prostate cancer. We find that 1,25(OH)(2)D(3) reduces c-Myc expression in multiple prostate epithelial cell lines, including C4-2 cells, an androgen-independent prostate cancer cell line. Reducing c-Myc expression to the levels observed after 1,25(OH)(2)D(3) treatment resulted in a comparable decrease in proliferation and G(1) accumulation demonstrating that down-regulation of c-Myc is a major component in the growth-inhibitory actions of 1,25(OH)2D(3). Treatment with 1,25(OH)(2)D(3) resulted in a 50% decrease in c-Myc mRNA but a much more extensive reduction in c-Myc protein. Treatment with 1,25(OH)(2)D(3) decreased c-Myc stability by increasing the proportion of c-Myc phosphorylated on T58, a glycogen synthase kinase-3beta site that serves as a signal for ubiquitin-mediated proteolysis. Thus, 1,25(OH)(2)D(3) reduces both c-Myc mRNA levels and c-Myc protein stability to inhibit growth of prostate cancer cells.
Figures
Similar articles
-
1α,25-dihydroxyvitamin D3 inhibits C4-2 prostate cancer cell growth via a retinoblastoma protein (Rb)-independent G1 arrest.Prostate. 2011 Jan 1;71(1):98-110. doi: 10.1002/pros.21226. Prostate. 2011. PMID: 20632309 Free PMC article.
-
The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells.Mol Cell Endocrinol. 2011 May 16;338(1-2):58-67. doi: 10.1016/j.mce.2011.02.023. Epub 2011 Mar 30. Mol Cell Endocrinol. 2011. PMID: 21458521 Free PMC article.
-
GADD45gamma: a new vitamin D-regulated gene that is antiproliferative in prostate cancer cells.Endocrinology. 2010 Oct;151(10):4654-64. doi: 10.1210/en.2010-0434. Epub 2010 Aug 25. Endocrinology. 2010. PMID: 20739400 Free PMC article.
-
The role of Vitamin D3 metabolism in prostate cancer.J Steroid Biochem Mol Biol. 2004 Nov;92(4):317-25. doi: 10.1016/j.jsbmb.2004.10.007. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663995 Review.
-
Vitamin D and systemic cancer: is this relevant to malignant melanoma?Br J Dermatol. 2002 Aug;147(2):197-213. doi: 10.1046/j.1365-2133.2002.04960.x. Br J Dermatol. 2002. PMID: 12174089 Review.
Cited by
-
Vitamin D and Hypoxia: Points of Interplay in Cancer.Cancers (Basel). 2022 Mar 31;14(7):1791. doi: 10.3390/cancers14071791. Cancers (Basel). 2022. PMID: 35406562 Free PMC article. Review.
-
Vitamin d, sunlight and prostate cancer risk.Adv Prev Med. 2011;2011:281863. doi: 10.4061/2011/281863. Epub 2011 Jun 8. Adv Prev Med. 2011. PMID: 21991434 Free PMC article.
-
A role for c-Myc in regulating anti-mycobacterial responses.Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17749-54. doi: 10.1073/pnas.1104892108. Epub 2011 Oct 12. Proc Natl Acad Sci U S A. 2011. PMID: 21997212 Free PMC article.
-
Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory.JBMR Plus. 2020 Sep 15;5(1):e10405. doi: 10.1002/jbm4.10405. eCollection 2021 Jan. JBMR Plus. 2020. PMID: 32904944 Free PMC article.
-
Nuclear targeting of cyclin-dependent kinase 2 reveals essential roles of cyclin-dependent kinase 2 localization and cyclin E in vitamin D-mediated growth inhibition.Endocrinology. 2010 Mar;151(3):896-908. doi: 10.1210/en.2009-1116. Epub 2010 Feb 10. Endocrinology. 2010. PMID: 20147522 Free PMC article.
References
-
- Masuda S, Jones G 2006 Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther 5:797–808 - PubMed
-
- Barreto AM, Schwartz GG, Woodruff R, Cramer SD 2000 25-Hydroxyvitamin D3, the prohormone of 1,25-dihydroxyvitamin D3, inhibits the proliferation of primary prostatic epithelial cells. Cancer Epidemiol Biomarkers Prev 9:265–270 - PubMed
-
- Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P 2000 Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 11:847–852 - PubMed
-
- John EM, Koo J, Schwartz GG 2007 Sun exposure and prostate cancer risk: evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev 16:1283–1286 - PubMed
-
- John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA 2005 Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res 65:5470–5479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

