Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells
- PMID: 19157638
- PMCID: PMC4737586
- DOI: 10.1016/j.imbio.2008.11.006
Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells
Abstract
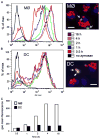
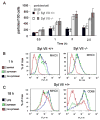
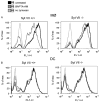
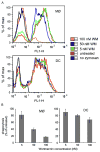
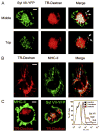
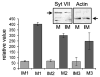
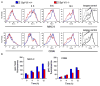
Synaptotagmin VII (Syt VII) is a Ca(2+) sensing molecule that regulates lysosomal exocytosis in several cell types. In macrophages (MØ), Syt VII is required for efficient uptake of large particle loads, by promoting the delivery of lysosomal membrane to phagocytic cups. Here we compare the phagocytic capacity of bone marrow-derived MØs and dendritic cells (DC), and show that the requirement for Syt VII correlates with the unique ability of MØs for continuous phagocytosis. In contrast to MØs, Syt VII(+/+) and Syt VII(-/-) immature DCs show similar levels of initial phagocytosis, followed by a marked decrease in particle uptake. [Ca(2+)](i) chelation and PI-3 kinase inhibition reduce particle uptake by MØs, but are markedly less inhibitory in DCs. Thus, immature DCs appear to lack the Syt VII, Ca(2+) and PI-3 kinase-dependent forms of phagocytosis that are present in MØs. Interestingly, expression of Syt VII is up-regulated during LPS-induced DC maturation, a stimulus that also induces Syt VII translocation from intracellular compartments to the plasma membrane. Syt VII(-/-) DCs show a delayed translocation of MHC class II to the cell surface during maturation, consistent with the possibility that Syt VII facilitates exocytosis and/or surface retention of molecules critical for antigen presentation.
Figures
Similar articles
-
Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes.J Cell Biol. 2006 Sep 25;174(7):997-1007. doi: 10.1083/jcb.200605004. Epub 2006 Sep 18. J Cell Biol. 2006. PMID: 16982801 Free PMC article.
-
Palmitoylation-dependent association with CD63 targets the Ca2+ sensor synaptotagmin VII to lysosomes.J Cell Biol. 2010 Nov 1;191(3):599-613. doi: 10.1083/jcb.201003021. J Cell Biol. 2010. PMID: 21041449 Free PMC article.
-
A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons.J Neurosci. 2006 Apr 26;26(17):4630-7. doi: 10.1523/JNEUROSCI.0009-06.2006. J Neurosci. 2006. PMID: 16641243 Free PMC article.
-
There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII.Trends Cell Biol. 2005 Nov;15(11):626-31. doi: 10.1016/j.tcb.2005.09.001. Epub 2005 Sep 15. Trends Cell Biol. 2005. PMID: 16168654 Review.
-
Synaptotagmin regulates mast cell functions.Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
Cited by
-
The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis.J Gen Physiol. 2018 Jun 4;150(6):783-807. doi: 10.1085/jgp.201711944. Epub 2018 May 24. J Gen Physiol. 2018. PMID: 29794152 Free PMC article. Review.
-
The Arabidopsis synaptotagmin SYTA regulates the cell-to-cell movement of diverse plant viruses.Front Plant Sci. 2014 Nov 6;5:584. doi: 10.3389/fpls.2014.00584. eCollection 2014. Front Plant Sci. 2014. PMID: 25414709 Free PMC article.
-
Hydrophobic contributions to the membrane docking of synaptotagmin 7 C2A domain: mechanistic contrast between isoforms 1 and 7.Biochemistry. 2012 Oct 2;51(39):7654-64. doi: 10.1021/bi3007115. Epub 2012 Sep 21. Biochemistry. 2012. PMID: 22966849 Free PMC article.
-
A Trypanosomatid Iron Transporter that Regulates Mitochondrial Function Is Required for Leishmania amazonensis Virulence.PLoS Pathog. 2016 Jan 7;12(1):e1005340. doi: 10.1371/journal.ppat.1005340. eCollection 2016 Jan. PLoS Pathog. 2016. PMID: 26741360 Free PMC article.
-
Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels.J Exp Med. 2013 Feb 11;210(2):401-16. doi: 10.1084/jem.20121368. Epub 2013 Feb 4. J Exp Med. 2013. PMID: 23382545 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

