A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia
- PMID: 19151713
- PMCID: PMC2697598
- DOI: 10.1038/ng.309
A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia
Abstract
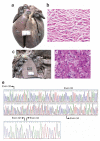
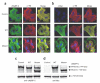
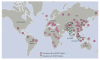
Heart failure is a leading cause of mortality in South Asians. However, its genetic etiology remains largely unknown. Cardiomyopathies due to sarcomeric mutations are a major monogenic cause for heart failure (MIM600958). Here, we describe a deletion of 25 bp in the gene encoding cardiac myosin binding protein C (MYBPC3) that is associated with heritable cardiomyopathies and an increased risk of heart failure in Indian populations (initial study OR = 5.3 (95% CI = 2.3-13), P = 2 x 10(-6); replication study OR = 8.59 (3.19-25.05), P = 3 x 10(-8); combined OR = 6.99 (3.68-13.57), P = 4 x 10(-11)) and that disrupts cardiomyocyte structure in vitro. Its prevalence was found to be high (approximately 4%) in populations of Indian subcontinental ancestry. The finding of a common risk factor implicated in South Asian subjects with cardiomyopathy will help in identifying and counseling individuals predisposed to cardiac diseases in this region.
Figures
Similar articles
-
Limited distribution of a cardiomyopathy-associated variant in India.Ann Hum Genet. 2010 Mar;74(2):184-8. doi: 10.1111/j.1469-1809.2010.00561.x. Epub 2010 Feb 18. Ann Hum Genet. 2010. PMID: 20201939 Free PMC article.
-
Role of common sarcomeric gene polymorphisms in genetic susceptibility to left ventricular dysfunction.J Genet. 2016 Jun;95(2):263-72. doi: 10.1007/s12041-016-0623-4. J Genet. 2016. PMID: 27350668
-
Unexpected myopathy associated with a mutation in MYBPC3 and misplacement of the cardiac myosin binding protein C.J Med Genet. 2010 Aug;47(8):575-7. doi: 10.1136/jmg.2009.072710. Epub 2009 Oct 26. J Med Genet. 2010. PMID: 19858127
-
Cardiac myosin-binding protein C (MYBPC3) in cardiac pathophysiology.Gene. 2015 Dec 1;573(2):188-97. doi: 10.1016/j.gene.2015.09.008. Epub 2015 Sep 8. Gene. 2015. PMID: 26358504 Free PMC article. Review.
-
Cardiomyopathy in Asian Cohorts: Genetic and Epigenetic Insights.Circ Genom Precis Med. 2023 Oct;16(5):496-506. doi: 10.1161/CIRCGEN.123.004079. Epub 2023 Aug 17. Circ Genom Precis Med. 2023. PMID: 37589150 Review.
Cited by
-
Genome-wide association studies of late-onset cardiovascular disease.J Mol Cell Cardiol. 2015 Jun;83:131-41. doi: 10.1016/j.yjmcc.2015.04.004. Epub 2015 Apr 11. J Mol Cell Cardiol. 2015. PMID: 25870159 Free PMC article. Review.
-
Reconstructing Indian population history.Nature. 2009 Sep 24;461(7263):489-94. doi: 10.1038/nature08365. Nature. 2009. PMID: 19779445 Free PMC article.
-
Advanced searching for hypertrophic cardiomyopathy heritability in real practice tomorrow.Front Cardiovasc Med. 2023 Jul 31;10:1236539. doi: 10.3389/fcvm.2023.1236539. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37583586 Free PMC article. Review.
-
Inactivation of Myosin binding protein C homolog in zebrafish as a model for human cardiac hypertrophy and diastolic dysfunction.J Am Heart Assoc. 2013 Sep 18;2(5):e000231. doi: 10.1161/JAHA.113.000231. J Am Heart Assoc. 2013. PMID: 24047589 Free PMC article.
-
Hypertrophic Cardiomyopathy: A Vicious Cycle Triggered by Sarcomere Mutations and Secondary Disease Hits.Antioxid Redox Signal. 2019 Aug 1;31(4):318-358. doi: 10.1089/ars.2017.7236. Epub 2018 Apr 11. Antioxid Redox Signal. 2019. PMID: 29490477 Free PMC article. Review.
References
-
- Reddy KS, Shah B, Varghese C, Ramadoss A. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1744–1749. - PubMed
-
- Joshi P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. J. Am. Med. Assoc. 2007;297:286–294. - PubMed
-
- Liew CC, Dzau VJ. Molecular genetics and genomics of heart failure. Nat. Rev. Genet. 2004;5:811–825. - PubMed
-
- Flavigny J, et al. Biomolecular interactions between human recombinant h-MyHC and cMyBP-Cs implicated in familial hypertrophic cardiomyopathy. Cardiovasc. Res. 2003;60:388–396. - PubMed
-
- Flavigny J, et al. COOH-terminal truncated cardiac myosin-binding protein C mutants resulting from familial hypertrophic cardiomyopathy mutations exhibit altered expression and/or incorporation in fetal rat cardiomyocytes. J. Mol. Biol. 1999;294:443–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

