Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization
- PMID: 19141863
- PMCID: PMC2727413
- DOI: 10.1182/blood-2008-10-184754
Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization
Abstract
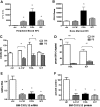
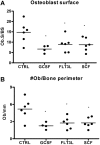
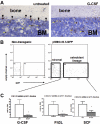
Current evidence suggests that hematopoietic stem/progenitor cell (HSPC) mobilization by granulocyte colony-stimulating factor (G-CSF) is mediated by induction of bone marrow proteases, attenuation of adhesion molecule function, and disruption of CXCL12/CXCR4 signaling in the bone marrow. The relative importance and extent to which these pathways overlap or function independently are uncertain. Despite evidence of protease activation in the bone marrow, HSPC mobilization by G-CSF or the chemokine Grobeta was abrogated in CXCR4(-/-) bone marrow chimeras. In contrast, HSPC mobilization by a VLA-4 antagonist was intact. To determine whether other mobilizing cytokines disrupt CXCR4 signaling, we characterized CXCR4 and CXCL12 expression after HSPC mobilization with Flt3 ligand (Flt3L) and stem cell factor (SCF). Indeed, treatment with Flt3L or SCF resulted in a marked decrease in CXCL12 expression in the bone marrow and a loss of surface expression of CXCR4 on HSPCs. RNA in situ and sorting experiments suggested that the decreased CXCL12 expression is secondary to a loss of osteoblast lineage cells. Collectively, these data suggest that disruption of CXCR4 signaling and attenuation of VLA-4 function are independent mechanisms of mobilization by G-CSF. Loss of CXCL12 expression by osteoblast appears to be a common and key step in cytokine-induced mobilization.
Figures
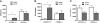
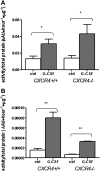
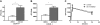
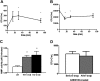
Comment in
-
HSC mobilization: new incites and insights.Blood. 2009 Aug 13;114(7):1283-4. doi: 10.1182/blood-2009-02-203240. Blood. 2009. PMID: 19679695 No abstract available.
Similar articles
-
Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice.J Exp Med. 2011 Feb 14;208(2):251-60. doi: 10.1084/jem.20101700. Epub 2011 Jan 31. J Exp Med. 2011. PMID: 21282380 Free PMC article.
-
Downregulation of CXCL12 signaling and altered hematopoietic stem and progenitor cell trafficking in a murine model of acute Anaplasma phagocytophilum infection.Innate Immun. 2012 Jun;18(3):418-28. doi: 10.1177/1753425911413794. Epub 2011 Sep 29. Innate Immun. 2012. PMID: 21964802 Free PMC article.
-
Adrenaline administration promotes the efficiency of granulocyte colony stimulating factor-mediated hematopoietic stem and progenitor cell mobilization in mice.Int J Hematol. 2013 Jan;97(1):50-7. doi: 10.1007/s12185-012-1228-1. Epub 2012 Dec 8. Int J Hematol. 2013. PMID: 23224606
-
Development of VLA4 and CXCR4 Antagonists for the Mobilization of Hematopoietic Stem and Progenitor Cells.Biomolecules. 2024 Aug 14;14(8):1003. doi: 10.3390/biom14081003. Biomolecules. 2024. PMID: 39199390 Free PMC article. Review.
-
Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4.Leukemia. 2012 Jan;26(1):34-53. doi: 10.1038/leu.2011.197. Epub 2011 Sep 2. Leukemia. 2012. PMID: 21886173 Free PMC article. Review.
Cited by
-
Granulocyte colony-stimulating factor does not enhance recruitment of bone marrow-derived cells in rats with acute myocardial infarction.Exp Clin Cardiol. 2012 Sep;17(3):83-8. Exp Clin Cardiol. 2012. PMID: 23620693 Free PMC article.
-
Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways.Cell Stem Cell. 2012 Aug 3;11(2):195-206. doi: 10.1016/j.stem.2012.04.024. Cell Stem Cell. 2012. PMID: 22862945 Free PMC article.
-
Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells.Exp Hematol. 2010 Apr;38(4):321-32. doi: 10.1016/j.exphem.2010.02.002. Epub 2010 Feb 12. Exp Hematol. 2010. PMID: 20153802 Free PMC article.
-
Cathepsin X is secreted by human osteoblasts, digests CXCL-12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts.Haematologica. 2010 Sep;95(9):1452-60. doi: 10.3324/haematol.2009.018671. Epub 2010 May 21. Haematologica. 2010. PMID: 20494937 Free PMC article.
-
Osteoclasts are dispensable for hematopoietic progenitor mobilization by granulocyte colony-stimulating factor in mice.Exp Hematol. 2015 Feb;43(2):110-4.e1-2. doi: 10.1016/j.exphem.2014.10.012. Epub 2014 Nov 1. Exp Hematol. 2015. PMID: 25461255 Free PMC article.
References
-
- Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A. Current trends in hematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374–2386. - PubMed
-
- Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–588. - PubMed
-
- Levesque JP, Winkler IG, Larsen SR, Rasko JE. Mobilization of bone marrow-derived progenitors. Handb Exp Pharmacol. 2007;180:3–36. - PubMed
-
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. - PubMed
-
- Lévesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

