NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance
- PMID: 19138055
- PMCID: PMC2659759
- DOI: 10.1667/RR1472.1
NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance
Abstract
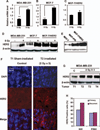
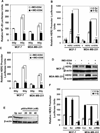
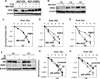
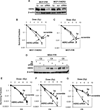
The molecular mechanisms governing acquired tumor resistance during radiotherapy remain to be elucidated. In breast cancer patients, overexpression of HER2 (human epidermal growth factor receptor 2) is correlated with aggressive tumor growth and increased recurrence. In the present study, we demonstrate that HER2 expression can be induced by radiation in breast cancer cells with a low basal level of HER2. Furthermore, HER2-postive tumors occur at a much higher frequency in recurrent invasive breast cancer (59%) compared to the primary tumors (41%). Interestingly, NF-kappaB is required for radiation-induced HER2 transactivation. HER2 was found to be co-activated with basal and radiation-induced NF-kappaB activity in radioresistant but not radiosensitive breast cancer cell lines after long-term radiation exposure, indicating that NF-kappaB-mediated HER2 overexpression is involved in radiation-induced repopulation in heterogeneous tumors. Finally, we found that inhibition of HER2 resensitizes the resistant cell lines to radiation. Since HER2 is shown to activate NF-kappaB, our data suggest a loop-like HER2-NF-kappaB-HER2 pathway in radiation-induced adaptive resistance in breast cancer cells.
Figures



Similar articles
-
Acquisition of stable inducible up-regulation of nuclear factor-kappaB by tumor necrosis factor exposure confers increased radiation resistance without increased transformation in breast cancer cells.Mol Cancer Res. 2008 Jan;6(1):78-88. doi: 10.1158/1541-7786.MCR-07-0339. Mol Cancer Res. 2008. PMID: 18234964
-
Trastuzumab-Resistant Luminal B Breast Cancer Cells Show Basal-Like Cell Growth Features Through NF-κB-Activation.Monoclon Antib Immunodiagn Immunother. 2016 Feb;35(1):1-11. doi: 10.1089/mab.2015.0056. Epub 2016 Jan 21. Monoclon Antib Immunodiagn Immunother. 2016. PMID: 26871511 Free PMC article.
-
Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells.Acta Biochim Biophys Sin (Shanghai). 2011 Aug;43(8):647-53. doi: 10.1093/abbs/gmr050. Epub 2011 Jun 28. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 21712253
-
Breast cancer adaptive resistance: HER2 and cancer stem cell repopulation in a heterogeneous tumor society.J Cancer Res Clin Oncol. 2014 Jan;140(1):1-14. doi: 10.1007/s00432-013-1494-1. Epub 2013 Aug 30. J Cancer Res Clin Oncol. 2014. PMID: 23990015 Free PMC article. Review.
-
NF-kappa B-mediated adaptive resistance to ionizing radiation.Free Radic Biol Med. 2008 Jan 1;44(1):1-13. doi: 10.1016/j.freeradbiomed.2007.09.022. Epub 2007 Oct 10. Free Radic Biol Med. 2008. PMID: 17967430 Free PMC article. Review.
Cited by
-
HER2 and breast cancer stem cells: more than meets the eye.Cancer Res. 2013 Jun 15;73(12):3489-93. doi: 10.1158/0008-5472.CAN-13-0260. Epub 2013 Jun 5. Cancer Res. 2013. PMID: 23740771 Free PMC article. Review.
-
HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab.Cancer Res. 2013 Mar 1;73(5):1635-46. doi: 10.1158/0008-5472.CAN-12-3349. Epub 2013 Feb 26. Cancer Res. 2013. PMID: 23442322 Free PMC article.
-
Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells.Nat Commun. 2020 Sep 14;11(1):4591. doi: 10.1038/s41467-020-18245-7. Nat Commun. 2020. PMID: 32929084 Free PMC article.
-
CDK1-Mediated SIRT3 Activation Enhances Mitochondrial Function and Tumor Radioresistance.Mol Cancer Ther. 2015 Sep;14(9):2090-102. doi: 10.1158/1535-7163.MCT-15-0017. Epub 2015 Jul 3. Mol Cancer Ther. 2015. PMID: 26141949 Free PMC article.
-
MyD88 signaling pathways: role in breast cancer.Front Oncol. 2024 Jan 29;14:1336696. doi: 10.3389/fonc.2024.1336696. eCollection 2024. Front Oncol. 2024. PMID: 38347830 Free PMC article. Review.
References
-
- Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat. Rev. Cancer 5. 2005;5:516–525. - PubMed
-
- Morgan WF. Genomic instability and bystander effects: a paradigm shift in radiation biology? Mil. Med. 2002;167:44–45. - PubMed
-
- Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Gius D. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. - PubMed
-
- Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, Fornace AJ., Jr Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64:6368–6371. - PubMed
-
- Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:781–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

