Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function
- PMID: 19129476
- PMCID: PMC2649274
- DOI: 10.1091/mbc.e08-09-0905
Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function
Abstract
Oxysterol-binding protein (OSBP) and OSBP-related proteins (ORPs) constitute a large gene family that differentially localize to organellar membranes, reflecting a functional role in sterol signaling and/or transport. OSBP partitions between the endoplasmic reticulum (ER) and Golgi apparatus where it imparts sterol-dependent regulation of ceramide transport and sphingomyelin synthesis. ORP9L also is localized to the ER-Golgi, but its role in secretion and lipid transport is unknown. Here we demonstrate that ORP9L partitioning between the trans-Golgi/trans-Golgi network (TGN), and the ER is mediated by a phosphatidylinositol 4-phosphate (PI-4P)-specific PH domain and VAMP-associated protein (VAP), respectively. In vitro, both OSBP and ORP9L mediated PI-4P-dependent cholesterol transport between liposomes, suggesting their primary in vivo function is sterol transfer between the Golgi and ER. Depletion of ORP9L by RNAi caused Golgi fragmentation, inhibition of vesicular somatitus virus glycoprotein transport from the ER and accumulation of cholesterol in endosomes/lysosomes. Complete cessation of protein transport and cell growth inhibition was achieved by inducible overexpression of ORP9S, a dominant negative variant lacking the PH domain. We conclude that ORP9 maintains the integrity of the early secretory pathway by mediating transport of sterols between the ER and trans-Golgi/TGN.
Figures

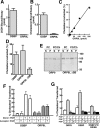
 ) or 25-hydroxycholesterol (2.5 μg/ml; ■) for 2 h followed by pulse-labeling with [3H]serine (10 μCi/ml) for 2 h. [3H]Serine-labeled SM, GlcCer and ceramide were extracted from cells, separated by thin-layer chromatography, and quantified as previously described (Perry and Ridgway, 2006). Results are the mean and SE for three separate experiments. (B) Expression of OSBP and ORP9L in CHO cells transiently transfected with control and targeting siRNAs. (C) CHO cells expressing ORP9L under the control of the Tet repressor were cultured in medium A without (−Dox) or with doxycycline (+Dox, 1 μg/ml) for 24 h. Cells then received solvent control (
) or 25-hydroxycholesterol (2.5 μg/ml; ■) for 2 h followed by pulse-labeling with [3H]serine (10 μCi/ml) for 2 h. [3H]Serine-labeled SM, GlcCer and ceramide were extracted from cells, separated by thin-layer chromatography, and quantified as previously described (Perry and Ridgway, 2006). Results are the mean and SE for three separate experiments. (B) Expression of OSBP and ORP9L in CHO cells transiently transfected with control and targeting siRNAs. (C) CHO cells expressing ORP9L under the control of the Tet repressor were cultured in medium A without (−Dox) or with doxycycline (+Dox, 1 μg/ml) for 24 h. Cells then received solvent control ( ) or 25OH (■) and were pulse-labeled with [3H]serine as described in A. Results are the mean and SEM for three separate experiments.
) or 25OH (■) and were pulse-labeled with [3H]serine as described in A. Results are the mean and SEM for three separate experiments.

Similar articles
-
Characterization of the sterol and phosphatidylinositol 4-phosphate binding properties of Golgi-associated OSBP-related protein 9 (ORP9).PLoS One. 2014 Sep 25;9(9):e108368. doi: 10.1371/journal.pone.0108368. eCollection 2014. PLoS One. 2014. PMID: 25255026 Free PMC article.
-
VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus.Exp Cell Res. 2004 Jul 15;297(2):533-47. doi: 10.1016/j.yexcr.2004.03.052. Exp Cell Res. 2004. PMID: 15212954
-
Oxysterol-binding Protein Activation at Endoplasmic Reticulum-Golgi Contact Sites Reorganizes Phosphatidylinositol 4-Phosphate Pools.J Biol Chem. 2016 Jan 15;291(3):1336-47. doi: 10.1074/jbc.M115.682997. Epub 2015 Nov 23. J Biol Chem. 2016. PMID: 26601944 Free PMC article.
-
Oxysterol-binding proteins: sterol and phosphoinositide sensors coordinating transport, signaling and metabolism.Prog Lipid Res. 2013 Oct;52(4):529-38. doi: 10.1016/j.plipres.2013.06.004. Epub 2013 Jul 2. Prog Lipid Res. 2013. PMID: 23830809 Review.
-
Molecular mechanisms and regulation of ceramide transport.Biochim Biophys Acta. 2005 Jun 1;1734(3):220-34. doi: 10.1016/j.bbalip.2005.04.001. Biochim Biophys Acta. 2005. PMID: 15907394 Review.
Cited by
-
The ALS8 protein VAPB interacts with the ER-Golgi recycling protein YIF1A and regulates membrane delivery into dendrites.EMBO J. 2013 Jul 17;32(14):2056-72. doi: 10.1038/emboj.2013.131. Epub 2013 Jun 4. EMBO J. 2013. PMID: 23736259 Free PMC article.
-
Molecular determinants of ER-Golgi contacts identified through a new FRET-FLIM system.J Cell Biol. 2019 Mar 4;218(3):1055-1065. doi: 10.1083/jcb.201812020. Epub 2019 Jan 18. J Cell Biol. 2019. PMID: 30659100 Free PMC article.
-
The diverse functions of oxysterol-binding proteins.Annu Rev Cell Dev Biol. 2010;26:157-77. doi: 10.1146/annurev.cellbio.042308.113334. Annu Rev Cell Dev Biol. 2010. PMID: 19575662 Free PMC article. Review.
-
Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network.Dev Cell. 2018 Nov 19;47(4):464-478.e8. doi: 10.1016/j.devcel.2018.10.012. Epub 2018 Nov 1. Dev Cell. 2018. PMID: 30393074 Free PMC article.
-
PI4P/PS countertransport by ORP10 at ER-endosome membrane contact sites regulates endosome fission.J Cell Biol. 2022 Jan 3;221(1):e202103141. doi: 10.1083/jcb.202103141. Epub 2021 Nov 24. J Cell Biol. 2022. PMID: 34817532 Free PMC article.
References
-
- Amarilio R., Ramachandran S., Sabanay H., Lev S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J. Biol. Chem. 2005;280:5934–5944. - PubMed
-
- Balla A., Tuymetova G., Tsiomenko A., Varnai P., Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. - PMC - PubMed
-
- Coxey R. A., Pentchev P. G., Campbell G., Blanchette-Mackie E. J. Differential accumulation of cholesterol in Golgi compartments of normal and Niemann-Pick type C fibroblasts incubated with LDL: a cytochemical freeze-fracture study. J. Lipid Res. 1993;34:1165–1176. - PubMed
-
- D'Angelo G., et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

