The Hsp90 mosaic: a picture emerges
- PMID: 19125165
- PMCID: PMC3339567
- DOI: 10.1038/nsmb0109-2
The Hsp90 mosaic: a picture emerges
Abstract
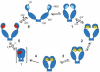
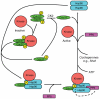
Hsp90s, molecular chaperones critically involved in many essential cellular processes, were the focus of a recent international conference held in Seeon, Germany. The scope of the conference ranged from structural and mechanistic insights all the way to medical applications.
Figures
Similar articles
-
Aha, another regulator for hsp90 chaperones.Mol Cell. 2002 Dec;10(6):1255-6. doi: 10.1016/s1097-2765(02)00793-1. Mol Cell. 2002. PMID: 12503997
-
Structure, function, and mechanism of the Hsp90 molecular chaperone.Adv Protein Chem. 2001;59:157-86. doi: 10.1016/s0065-3233(01)59005-1. Adv Protein Chem. 2001. PMID: 11868271 Review. No abstract available.
-
Constantly updated knowledge of Hsp90.J Biochem. 2005 Apr;137(4):443-7. doi: 10.1093/jb/mvi056. J Biochem. 2005. PMID: 15858167 Review.
-
Structure, function and regulation of the hsp90 machinery.Biomed J. 2013 May-Jun;36(3):106-17. doi: 10.4103/2319-4170.113230. Biomed J. 2013. PMID: 23806880 Review.
-
Detecting HSP90 phosphorylation.Methods Mol Biol. 2011;787:67-74. doi: 10.1007/978-1-61779-295-3_5. Methods Mol Biol. 2011. PMID: 21898227 Free PMC article.
Cited by
-
A detailed modular analysis of heat-shock protein dynamics under acute and chronic stress and its implication in anxiety disorders.PLoS One. 2012;7(8):e42958. doi: 10.1371/journal.pone.0042958. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22937003 Free PMC article.
-
Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development.Signal Transduct Target Ther. 2020 Jul 13;5(1):125. doi: 10.1038/s41392-020-00233-4. Signal Transduct Target Ther. 2020. PMID: 32661235 Free PMC article. Review.
-
Phosphorylated and unphosphorylated serine 13 of CDC37 stabilize distinct interactions between its client and HSP90 binding domains.Biochemistry. 2015 Feb 24;54(7):1493-504. doi: 10.1021/bi501129g. Epub 2015 Feb 11. Biochemistry. 2015. PMID: 25619116 Free PMC article.
-
Two closed ATP- and ADP-dependent conformations in yeast Hsp90 chaperone detected by Mn(II) EPR spectroscopic techniques.Proc Natl Acad Sci U S A. 2020 Jan 7;117(1):395-404. doi: 10.1073/pnas.1916030116. Epub 2019 Dec 20. Proc Natl Acad Sci U S A. 2020. PMID: 31862713 Free PMC article.
-
In Vivo Conformational Dynamics of Hsp90 and Its Interactors.Cell Chem Biol. 2016 Jun 23;23(6):716-26. doi: 10.1016/j.chembiol.2016.05.012. Cell Chem Biol. 2016. PMID: 27341434 Free PMC article.
References
-
- Pratt WB, Toft DO. Exp. Biol. Med. (Maywood) 2003;228:111–133. - PubMed
-
- Shiau AK, Harris SF, Southworth DR, Agard DA. Cell. 2006;127:329–340. - PubMed
-
- Leskovar A, Wegele H, Werbeck ND, Buchner J, Reinstein J. J. Biol. Chem. 2008;283:11677–11688. - PubMed
-
- Siligardi G, et al. J. Biol. Chem. 2004;279:51989–51998. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

