USP7 counteracts SCFbetaTrCP- but not APCCdh1-mediated proteolysis of Claspin
- PMID: 19124652
- PMCID: PMC2615094
- DOI: 10.1083/jcb.200807137
USP7 counteracts SCFbetaTrCP- but not APCCdh1-mediated proteolysis of Claspin
Abstract
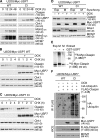
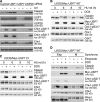
Claspin is an adaptor protein that facilitates the ataxia telangiectasia and Rad3-related (ATR)-mediated phosphorylation and activation of Chk1, a key effector kinase in the DNA damage response. Efficient termination of Chk1 signaling in mitosis and during checkpoint recovery requires SCF(betaTrCP)-dependent destruction of Claspin. Here, we identify the deubiquitylating enzyme ubiquitin-specific protease 7 (USP7) as a novel regulator of Claspin stability. Claspin and USP7 interact in vivo, and USP7 is required to maintain steady-state levels of Claspin. Furthermore, USP7-mediated deubiquitylation markedly prolongs the half-life of Claspin, which in turn increases the magnitude and duration of Chk1 phosphorylation in response to genotoxic stress. Finally, we find that in addition to the M phase-specific, SCF(betaTrCP)-mediated degradation, Claspin is destabilized by the anaphase-promoting complex (APC) and thus remains unstable in G1. Importantly, we demonstrate that USP7 specifically opposes the SCF(betaTrCP)- but not APC(Cdh1)-mediated degradation of Claspin. Thus, Claspin turnover is controlled by multiple ubiquitylation and deubiquitylation activities, which together provide a flexible means to regulate the ATR-Chk1 pathway.
Figures


Similar articles
-
USP7 controls Chk1 protein stability by direct deubiquitination.Cell Cycle. 2014;13(24):3921-6. doi: 10.4161/15384101.2014.973324. Cell Cycle. 2014. PMID: 25483066 Free PMC article.
-
Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress.Mol Cell. 2006 Aug 4;23(3):307-18. doi: 10.1016/j.molcel.2006.06.016. Mol Cell. 2006. PMID: 16885021
-
SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response.Mol Cell. 2006 Aug 4;23(3):319-29. doi: 10.1016/j.molcel.2006.06.013. Mol Cell. 2006. PMID: 16885022
-
Regulation of ATR-CHK1 signaling by ubiquitination of CLASPIN.Biochem Soc Trans. 2022 Oct 31;50(5):1471-1480. doi: 10.1042/BST20220729. Biochem Soc Trans. 2022. PMID: 36196914 Review.
-
Claspin - checkpoint adaptor and DNA replication factor.FEBS J. 2019 Feb;286(3):441-455. doi: 10.1111/febs.14594. Epub 2018 Jun 29. FEBS J. 2019. PMID: 29931808 Review.
Cited by
-
USP29 controls the stability of checkpoint adaptor Claspin by deubiquitination.Oncogene. 2015 Feb 19;34(8):1058-63. doi: 10.1038/onc.2014.38. Epub 2014 Mar 17. Oncogene. 2015. PMID: 24632611
-
Coordinating DNA Replication and Mitosis through Ubiquitin/SUMO and CDK1.Int J Mol Sci. 2021 Aug 16;22(16):8796. doi: 10.3390/ijms22168796. Int J Mol Sci. 2021. PMID: 34445496 Free PMC article. Review.
-
Active site-targeted covalent irreversible inhibitors of USP7 impair the functions of Foxp3+ T-regulatory cells by promoting ubiquitination of Tip60.PLoS One. 2017 Dec 13;12(12):e0189744. doi: 10.1371/journal.pone.0189744. eCollection 2017. PLoS One. 2017. PMID: 29236775 Free PMC article.
-
Deubiquitinating Enzyme-Mediated Signaling Networks in Cancer Stem Cells.Cancers (Basel). 2020 Nov 4;12(11):3253. doi: 10.3390/cancers12113253. Cancers (Basel). 2020. PMID: 33158118 Free PMC article. Review.
-
Ubiquitin-specific protease 7 as a potential therapeutic target in dogs with hematopoietic malignancies.J Vet Intern Med. 2021 Mar;35(2):1041-1051. doi: 10.1111/jvim.16082. Epub 2021 Mar 2. J Vet Intern Med. 2021. PMID: 33650720 Free PMC article.
References
-
- Bartek, J., and J. Lukas. 2007. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 19:238–245. - PubMed
-
- Busino, L., M. Donzelli, M. Chiesa, D. Guardavaccaro, D. Ganoth, N.V. Dorrello, A. Hershko, M. Pagano, and G.F. Draetta. 2003. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 426:87–91. - PubMed
-
- Canning, M., C. Boutell, J. Parkinson, and R.D. Everett. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160–38168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

