Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies?
- PMID: 19120495
- PMCID: PMC2632709
- DOI: 10.1111/j.1365-2567.2008.03010.x
Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies?
Erratum in
- Immunology. 2009 Mar;126(3):446
Abstract
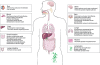
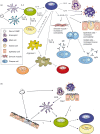
Although the molecules and cells involved in triggering immune responses against parasitic worms (helminths) remain enigmatic, research has continued to implicate expansions of T-helper type 2 (Th2) cells and regulatory T-helper (T(reg)) cells as a characteristic response to these organisms. An intimate association has also emerged between Th2 responses and wound-healing functions. As helminth infections in humans are associated with a strong Th2/T(reg) immunoregulatory footprint (often termed a 'modified Th2' response), plausible links have been made to increased susceptibility to microbial pathogens in helminth-infected populations in the tropics and to the breakdowns in immunological control (allergy and autoimmunity) that are increasing in frequency in helminth-free developed countries. Removal of helminths and their anti-inflammatory influence may also have hazards for populations exposed to infectious agents, such as malaria and influenza, whose worst effects are mediated by excessive inflammatory reactions. The patterns seen in the control of helminth immunity are discussed from an evolutionary perspective. Whilst an inability to correctly regulate the immune system in the absence of helminth infection might seem highly counter-adaptive, the very ancient and pervasive relationship between vertebrates and helminths supports a view that immunological control networks have been selected to function within the context of a modified Th2 environment. The absence of immunoregulatory stimuli from helminths may therefore uncover maladaptations that were not previously exposed to selection.
Figures

Similar articles
-
What Can Parasites Tell Us About the Pathogenesis and Treatment of Asthma and Allergic Diseases.Front Immunol. 2020 Sep 11;11:2106. doi: 10.3389/fimmu.2020.02106. eCollection 2020. Front Immunol. 2020. PMID: 33013887 Free PMC article. Review.
-
Regulation of allergy and autoimmunity in helminth infection.Clin Rev Allergy Immunol. 2004 Feb;26(1):35-50. doi: 10.1385/CRIAI:26:1:35. Clin Rev Allergy Immunol. 2004. PMID: 14755074 Review.
-
Mapping immune response profiles: the emerging scenario from helminth immunology.Eur J Immunol. 2007 Dec;37(12):3319-26. doi: 10.1002/eji.200737765. Eur J Immunol. 2007. PMID: 18000958 Review.
-
Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases.Immunol Rev. 2014 May;259(1):206-30. doi: 10.1111/imr.12164. Immunol Rev. 2014. PMID: 24712468 Review.
-
The tropics, helminth infections and hygiene hypotheses.Expert Rev Clin Immunol. 2018 Feb;14(2):99-102. doi: 10.1080/1744666X.2018.1424543. Epub 2018 Jan 10. Expert Rev Clin Immunol. 2018. PMID: 29300114 Review.
Cited by
-
Intestinal protozoan infections shape fecal bacterial microbiota in children from Guinea-Bissau.PLoS Negl Trop Dis. 2021 Mar 3;15(3):e0009232. doi: 10.1371/journal.pntd.0009232. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33657123 Free PMC article.
-
Reproductive trade-offs in extant hunter-gatherers suggest adaptive mechanism for the Neolithic expansion.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):4694-9. doi: 10.1073/pnas.1524031113. Epub 2016 Apr 11. Proc Natl Acad Sci U S A. 2016. PMID: 27071109 Free PMC article.
-
Changing expression of vertebrate immunity genes in an anthropogenic environment: a controlled experiment.BMC Evol Biol. 2016 Sep 1;16(1):175. doi: 10.1186/s12862-016-0751-8. BMC Evol Biol. 2016. PMID: 27586387 Free PMC article.
-
Immunomodulation of Murine Chronic DSS-Induced Colitis by Tuftsin-Phosphorylcholine.J Clin Med. 2019 Dec 26;9(1):65. doi: 10.3390/jcm9010065. J Clin Med. 2019. PMID: 31888063 Free PMC article.
-
The evolution of powerful yet perilous immune systems.Trends Immunol. 2022 Feb;43(2):117-131. doi: 10.1016/j.it.2021.12.002. Epub 2021 Dec 20. Trends Immunol. 2022. PMID: 34949534 Free PMC article. Review.
References
-
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. - PubMed
-
- Anderson RC. Why do fish have so few roundworm (nematode) parasites? Environ Biol Fishes. 1996;46:1–5.
-
- Littlewood DTJ, Rohde K, Clough KA. The interrelationships of all major groups of Platyhelminthes: phylogenetic evidence from morphology and molecules. Biol J Linn Soc Lond. 1999;66:75–114.
-
- Clark WC. Origins of the parasitic habit in the Nematoda. Int J Parasitol. 1994;24:1117–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

