G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation
- PMID: 19118199
- PMCID: PMC2613041
- DOI: 10.1073/pnas.0811685106
G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation
Abstract
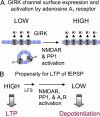
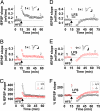
Excitatory synapses in the brain undergo activity-dependent changes in the strength of synaptic transmission. Such synaptic plasticity as exemplified by long-term potentiation (LTP) is considered a cellular correlate of learning and memory. The presence of G protein-activated inwardly rectifying K(+) (GIRK) channels near excitatory synapses on dendritic spines suggests their possible involvement in synaptic plasticity. However, whether activity-dependent regulation of GIRK channels affects excitatory synaptic plasticity is unknown. In a companion article we have reported activity-dependent regulation of GIRK channel density in cultured hippocampal neurons that requires activity of NMDA receptors (NMDAR) and protein phosphatase-1 (PP1) and takes place within 15 min. In this study, we performed whole-cell recordings of cultured hippocampal neurons and found that NMDAR activation increases basal GIRK current and GIRK channel activation mediated by adenosine A(1) receptors, but not GABA(B) receptors. Given the similar involvement of NMDARs, adenosine A(1) receptors, and PP1 in depotentiation of LTP caused by low-frequency stimulation that immediately follows LTP-inducing high-frequency stimulation, we wondered whether NMDAR-induced increase in GIRK channel surface density and current may contribute to the molecular mechanisms underlying this specific depotentiation. Remarkably, GIRK2 null mutation or GIRK channel blockade abolishes depotentiation of LTP, demonstrating that GIRK channels are critical for depotentiation, one form of excitatory synaptic plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
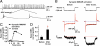
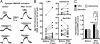

Similar articles
-
G-Protein-Gated Inwardly Rectifying Potassium (Kir3/GIRK) Channels Govern Synaptic Plasticity That Supports Hippocampal-Dependent Cognitive Functions in Male Mice.J Neurosci. 2021 Aug 18;41(33):7086-7102. doi: 10.1523/JNEUROSCI.2849-20.2021. Epub 2021 Jul 14. J Neurosci. 2021. PMID: 34261700 Free PMC article.
-
Hippocampal long-term synaptic depression and memory deficits induced in early amyloidopathy are prevented by enhancing G-protein-gated inwardly rectifying potassium channel activity.J Neurochem. 2020 May;153(3):362-376. doi: 10.1111/jnc.14946. Epub 2020 Jan 30. J Neurochem. 2020. PMID: 31875959 Free PMC article.
-
G Protein-Gated K+ Channel Ablation in Forebrain Pyramidal Neurons Selectively Impairs Fear Learning.Biol Psychiatry. 2016 Nov 15;80(10):796-806. doi: 10.1016/j.biopsych.2015.10.004. Epub 2015 Nov 10. Biol Psychiatry. 2016. PMID: 26612516 Free PMC article.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
LTD, LTP, and the sliding threshold for long-term synaptic plasticity.Hippocampus. 1996;6(1):35-42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. Hippocampus. 1996. PMID: 8878740 Review.
Cited by
-
Molecular basis of the facilitation of the heterooligomeric GIRK1/GIRK4 complex by cAMP dependent protein kinase.Biochim Biophys Acta. 2013 Apr;1828(4):1214-21. doi: 10.1016/j.bbamem.2012.12.016. Epub 2013 Jan 7. Biochim Biophys Acta. 2013. PMID: 23305758 Free PMC article.
-
Overexpression of KCNJ3 gene splice variants affects vital parameters of the malignant breast cancer cell line MCF-7 in an opposing manner.BMC Cancer. 2016 Aug 12;16:628. doi: 10.1186/s12885-016-2664-8. BMC Cancer. 2016. PMID: 27519272 Free PMC article.
-
Adenosine A1 Receptor mRNA Expression by Neurons and Glia in the Auditory Forebrain.Anat Rec (Hoboken). 2018 Nov;301(11):1882-1905. doi: 10.1002/ar.23907. Epub 2018 Oct 12. Anat Rec (Hoboken). 2018. PMID: 30315630 Free PMC article.
-
Neuronal G protein-gated K+ channels.Am J Physiol Cell Physiol. 2022 Aug 1;323(2):C439-C460. doi: 10.1152/ajpcell.00102.2022. Epub 2022 Jun 15. Am J Physiol Cell Physiol. 2022. PMID: 35704701 Free PMC article. Review.
-
Constructing a road map from synapses to behaviour. Meeting on Synapses: From Molecules to Circuits & Behavior.EMBO Rep. 2009 Sep;10(9):958-62. doi: 10.1038/embor.2009.187. Epub 2009 Aug 7. EMBO Rep. 2009. PMID: 19662076 Free PMC article. No abstract available.
References
-
- Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. - PubMed
-
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. - PubMed
-
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann NY Acad Sci. 1999;868:515–525. - PubMed
-
- Staubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

