CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis
- PMID: 19115422
- PMCID: PMC2853874
- DOI: 10.1002/hep.22743
CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis
Abstract
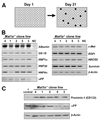
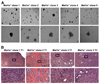
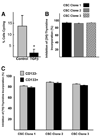
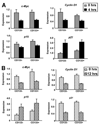
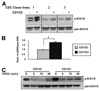
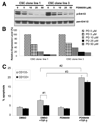
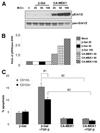
Methionine adenosyltransferase (MAT) is an essential enzyme required for S-adenosylmethionine biosynthesis. Hepatic MAT activity falls during chronic liver injury, and mice lacking Mat1a develop spontaneous hepatocellular carcinoma by 18 months. We have previously demonstrated that CD133(+)CD45(-) oval cells isolated from 16-month-old Mat1a(-/-) mice represent a liver cancer stem cell population. The transforming growth factor beta (TGF-beta) pathway constitutes a central signaling network in proliferation, apoptosis, and tumorigenesis. In this study, we tested the response of tumorigenic liver stem cells to TGF-beta. CD133(+)CD45(-) oval cells were isolated from premalignant 16-month-old Mat1a(-/-) mice by flow cytometry and expanded as five clone lines derived from a single cell. All clone lines demonstrated expression of both hepatocyte and cholangiocyte markers and maintained a small population (0.5% to 2%) of CD133(+) cells in vitro, and three of five clone lines produced tumors. Although TGF-beta1 inhibited cell growth equally in CD133(-) and CD133(+) cells from each clone line, the CD133(+) population demonstrated significant resistance to TGF-beta-induced apoptosis compared with CD133(-) cells. Furthermore, CD133(+) cells demonstrated a substantial increase in mitogen-activated protein kinase (MAPK) pathway activation, as demonstrated by phosphorylated extracellular signal-regulated kinase levels before and after TGF-beta stimulation. MAPK inhibition using mitogen-activated protein kinase kinase 1 (MEK1) inhibitor PD98059 led to a significant increase in TGF-beta-induced apoptosis in CD133(+) cells. Conversely, a constitutively active form of MEK1 blocked the apoptotic effects of TGF-beta in CD133(-) cells.
Conclusion: CD133(+) liver cancer stem cells exhibit relative resistance to TGF-beta-induced apoptosis. One mechanism of resistance to TGF-beta-induced apoptosis in CD133(+) cancer stem cells is an activated mitogen-activated protein kinase/extracellular signal-regulated kinase pathway.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

Similar articles
-
Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice.Hepatology. 2008 Apr;47(4):1288-97. doi: 10.1002/hep.22141. Hepatology. 2008. PMID: 18167064 Free PMC article.
-
Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice.Stem Cells. 2009 Feb;27(2):290-9. doi: 10.1634/stemcells.2008-0332. Stem Cells. 2009. PMID: 19008348 Free PMC article.
-
CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling.Hepatology. 2012 Mar;55(3):807-20. doi: 10.1002/hep.24739. Epub 2012 Jan 13. Hepatology. 2012. PMID: 21994122
-
Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis.J Hepatol. 2013 Oct;59(4):830-41. doi: 10.1016/j.jhep.2013.04.031. Epub 2013 May 7. J Hepatol. 2013. PMID: 23665184 Review.
-
Hepatic stem cells and transforming growth factor β in hepatocellular carcinoma.Nat Rev Gastroenterol Hepatol. 2012 Sep;9(9):530-8. doi: 10.1038/nrgastro.2012.114. Epub 2012 Jun 19. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22710573 Free PMC article. Review.
Cited by
-
Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta.Hepatology. 2010 May;51(5):1635-44. doi: 10.1002/hep.23544. Hepatology. 2010. PMID: 20196115 Free PMC article.
-
Isolation of CD133+ liver stem cells for clonal expansion.J Vis Exp. 2011 Oct 10;(56):3183. doi: 10.3791/3183. J Vis Exp. 2011. PMID: 22006186 Free PMC article.
-
Cellular reprogramming and hepatocellular carcinoma development.World J Gastroenterol. 2013 Dec 21;19(47):8850-60. doi: 10.3748/wjg.v19.i47.8850. World J Gastroenterol. 2013. PMID: 24379607 Free PMC article. Review.
-
TGF-β: duality of function between tumor prevention and carcinogenesis.J Natl Cancer Inst. 2014 Feb;106(2):djt369. doi: 10.1093/jnci/djt369. J Natl Cancer Inst. 2014. PMID: 24511106 Free PMC article. Review.
-
Inhibition of oxidative stress-elicited AKT activation facilitates PPARγ agonist-mediated inhibition of stem cell character and tumor growth of liver cancer cells.PLoS One. 2013 Aug 30;8(8):e73038. doi: 10.1371/journal.pone.0073038. eCollection 2013. PLoS One. 2013. PMID: 24023668 Free PMC article.
References
-
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. - PubMed
-
- Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7–11. - PubMed
-
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. - PubMed
-
- Gou S, Liu T, Wang C, Yin T, Li K, Yang M, et al. Establishment of clonal colony-forming assay for propagation of pancreatic cancer cells with stem cell properties. Pancreas. 2007;34:429–435. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
