Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1
- PMID: 19112176
- PMCID: PMC2658102
- DOI: 10.1074/jbc.M808064200
Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1
Abstract
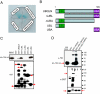
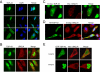
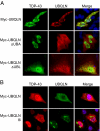
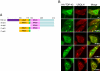
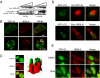
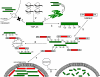
TDP-43 (43-kDa TAR DNA-binding domain protein) is a major constituent of ubiquitin-positive cytoplasmic aggregates present in neurons of patients with fronto-temporal lobular dementia and amyotrophic lateral sclerosis (ALS). The pathologic significance of TDP-43 aggregation is not known; however, dominant mutations in TDP-43 cause a subset of ALS cases, suggesting that misfolding and/or altered trafficking of TDP-43 is relevant to the disease process. Here, we show that the presenilin-binding protein ubiquilin 1 (UBQLN) plays a role in TDP-43 aggregation. TDP-43 interacted with UBQLN both in yeast and in vitro, and the carboxyl-terminal ubiquitin-associated domain of UBQLN was both necessary and sufficient for binding to polyubiquitylated forms of TDP-43. Overexpression of UBQLN recruited TDP-43 to detergent-resistant cytoplasmic aggregates that colocalized with the autophagosomal marker, LC3. UBQLN-dependent aggregation required the UBQLN UBA domain, was mediated by non-overlapping regions of TDP-43, and was abrogated by a mutation in UBQLN previously linked to Alzheimer disease. Four ALS-associated alleles of TDP-43 also coaggregated with UBQLN, and the extent of aggregation correlated with in vitro UBQLN binding affinity. Our findings suggest that UBQLN is a polyubiquitin-TDP-43 cochaperone that mediates the autophagosomal delivery and/or proteasome targeting of TDP-43 aggregates.
Figures

Similar articles
-
Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion.Acta Neuropathol. 2012 Jun;123(6):825-39. doi: 10.1007/s00401-012-0970-z. Epub 2012 Mar 18. Acta Neuropathol. 2012. PMID: 22426854 Free PMC article.
-
Mutation-dependent aggregation and toxicity in a Drosophila model for UBQLN2-associated ALS.Hum Mol Genet. 2018 Jan 15;27(2):322-337. doi: 10.1093/hmg/ddx403. Hum Mol Genet. 2018. PMID: 29161404 Free PMC article.
-
Depletion of Ubiquilin induces an augmentation in soluble ubiquitinated Drosophila TDP-43 to drive neurotoxicity in the fly.Biochim Biophys Acta Mol Basis Dis. 2018 Sep;1864(9 Pt B):3038-3049. doi: 10.1016/j.bbadis.2018.06.017. Epub 2018 Jun 21. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29936333
-
UBQLN proteins in health and disease with a focus on UBQLN2 in ALS/FTD.FEBS J. 2022 Oct;289(20):6132-6153. doi: 10.1111/febs.16129. Epub 2021 Jul 28. FEBS J. 2022. PMID: 34273246 Free PMC article. Review.
-
[Molecular mechanism of amyotrophic lateral sclerosis (ALS) from the viewpoint of the formation and degeneration of transactive response DNA-binding protein 43 kDa (TDP-43) inclusions].Rinsho Shinkeigaku. 2020 Feb 27;60(2):109-116. doi: 10.5692/clinicalneurol.cn-001362. Epub 2020 Jan 19. Rinsho Shinkeigaku. 2020. PMID: 31956195 Review. Japanese.
Cited by
-
Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration.Nat Rev Neurosci. 2011 Nov 30;13(1):38-50. doi: 10.1038/nrn3121. Nat Rev Neurosci. 2011. PMID: 22127299 Free PMC article. Review.
-
Ushering in the cardiac role of Ubiquilin1.J Clin Invest. 2018 Dec 3;128(12):5195-5197. doi: 10.1172/JCI124567. Epub 2018 Oct 22. J Clin Invest. 2018. PMID: 30352427 Free PMC article.
-
The ubiquitin proteasome system in neuropathology.Acta Neuropathol. 2009 Sep;118(3):329-47. doi: 10.1007/s00401-009-0560-x. Epub 2009 Jul 14. Acta Neuropathol. 2009. PMID: 19597829 Free PMC article. Review.
-
Invertebrate genetic models of amyotrophic lateral sclerosis.Front Mol Neurosci. 2024 Mar 4;17:1328578. doi: 10.3389/fnmol.2024.1328578. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38500677 Free PMC article. Review.
-
Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease.Transl Res. 2019 Feb;204:19-30. doi: 10.1016/j.trsl.2018.10.002. Epub 2018 Oct 12. Transl Res. 2019. PMID: 30391475 Free PMC article. Review.
References
-
- Mitsumoto, H., Chad, D. A., and Pioro, E. P. (1998) Amyotrophic Lateral Sclerosis, Davis, Philadelphia, PA
-
- Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362 59-62 - PubMed
-
- Andersen, P. M., Sims, K. B., Xin, W. W., Kiely, R., O'Neill, G., Ravits, J., Pioro, E., Harati, Y., Brower, R. D., Levine, J. S., Heinicke, H. U., Seltzer, W., Boss, M., and Brown, R. H., Jr. (2003) Amyotroph. Lateral Scler. Other Motor Neuron Disord. 4 62-73 - PubMed
-
- Boillee, S., Vande Velde, C., and Cleveland, D. W. (2006) Neuron 52 39-59 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

