Germline competent embryonic stem cells derived from rat blastocysts
- PMID: 19109898
- PMCID: PMC2735113
- DOI: 10.1016/j.cell.2008.12.006
Germline competent embryonic stem cells derived from rat blastocysts
Abstract
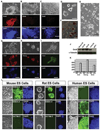
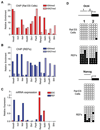
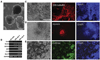
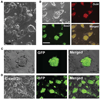
Rats have important advantages over mice as an experimental system for physiological and pharmacological investigations. The lack of rat embryonic stem (ES) cells has restricted the availability of transgenic technologies to create genetic models in this species. Here, we show that rat ES cells can be efficiently derived, propagated, and genetically manipulated in the presence of small molecules that specifically inhibit GSK3, MEK, and FGF receptor tyrosine kinases. These rat ES cells express pluripotency markers and retain the capacity to differentiate into derivatives of all three germ layers. Most importantly, they can produce high rates of chimerism when reintroduced into early stage embryos and can transmit through the germline. Establishment of authentic rat ES cells will make possible sophisticated genetic manipulation to create models for the study of human diseases.
Figures

Similar articles
-
Capture of authentic embryonic stem cells from rat blastocysts.Cell. 2008 Dec 26;135(7):1287-98. doi: 10.1016/j.cell.2008.12.007. Cell. 2008. PMID: 19109897
-
Effect of different culture conditions on establishment of embryonic stem cells from BALB/cAJ and NZB/BINJ mice.Cell Reprogram. 2010 Dec;12(6):679-88. doi: 10.1089/cell.2010.0018. Epub 2010 Oct 26. Cell Reprogram. 2010. PMID: 20977302
-
Establishment of embryonic stem cells from rat blastocysts.Methods Mol Biol. 2010;597:169-77. doi: 10.1007/978-1-60327-389-3_12. Methods Mol Biol. 2010. PMID: 20013233
-
In or out stemness: comparing growth factor signalling in mouse embryonic stem cells and primordial germ cells.Curr Stem Cell Res Ther. 2009 May;4(2):87-97. doi: 10.2174/157488809788167391. Curr Stem Cell Res Ther. 2009. PMID: 19442193 Review.
-
Gene-manipulated embryonic stem cells for rat transgenesis.Cell Mol Life Sci. 2011 Jun;68(11):1911-5. doi: 10.1007/s00018-011-0669-7. Epub 2011 Mar 25. Cell Mol Life Sci. 2011. PMID: 21437643 Free PMC article. Review.
Cited by
-
Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming.Nat Commun. 2016 Mar 31;7:11124. doi: 10.1038/ncomms11124. Nat Commun. 2016. PMID: 27030341 Free PMC article.
-
Comparative Analysis of piggyBac, CRISPR/Cas9 and TALEN Mediated BAC Transgenesis in the Zygote for the Generation of Humanized SIRPA Rats.Sci Rep. 2016 Aug 17;6:31455. doi: 10.1038/srep31455. Sci Rep. 2016. PMID: 27530248 Free PMC article.
-
Recombinant rabbit leukemia inhibitory factor and rabbit embryonic fibroblasts support the derivation and maintenance of rabbit embryonic stem cells.Cell Reprogram. 2012 Aug;14(4):364-76. doi: 10.1089/cell.2012.0001. Epub 2012 Jul 9. Cell Reprogram. 2012. PMID: 22775411 Free PMC article.
-
Derivation of Porcine Embryonic Stem-Like Cells from In Vitro-Produced Blastocyst-Stage Embryos.Sci Rep. 2016 May 13;6:25838. doi: 10.1038/srep25838. Sci Rep. 2016. PMID: 27173828 Free PMC article.
-
BMP4 acts as a dorsal telencephalic morphogen in a mouse embryonic stem cell culture system.Biol Open. 2016 Dec 15;5(12):1834-1843. doi: 10.1242/bio.012021. Biol Open. 2016. PMID: 27815243 Free PMC article.
References
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Brenin D, Look J, Bader M, Hubner N, Levan G, Iannaccone P. Rat embryonic stem cells: a progress report. Transplant. Proc. 1997;29:1761–1765. - PubMed
-
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
-
- Buehr M, Nichols J, Stenhouse F, Mountford P, Greenhalgh CJ, Kantachuvesiri S, Brooker G, Mullins J, Smith AG. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol. Reprod. 2003;68:222–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

