Orthopoxviruses require a functional ubiquitin-proteasome system for productive replication
- PMID: 19109393
- PMCID: PMC2643736
- DOI: 10.1128/JVI.01753-08
Orthopoxviruses require a functional ubiquitin-proteasome system for productive replication
Abstract
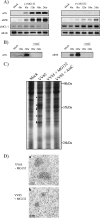
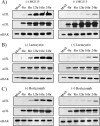
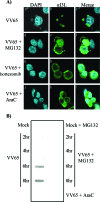
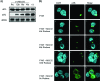
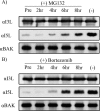
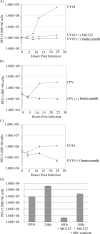
Cellular homeostasis depends on an intricate balance of protein expression and degradation. The ubiquitin-proteasome pathway plays a crucial role in specifically targeting proteins tagged with ubiquitin for destruction. This degradation can be effectively blocked by both chemically synthesized and natural proteasome inhibitors. Poxviruses encode a number of proteins that exploit the ubiquitin-proteasome system, including virally encoded ubiquitin molecules and ubiquitin ligases, as well as BTB/kelch proteins and F-box proteins, which interact with cellular ubiquitin ligases. Here we show that poxvirus infection was dramatically affected by a range of proteasome inhibitors, including MG132, MG115, lactacystin, and bortezomib (Velcade). Confocal microscopy demonstrated that infected cells treated with MG132 or bortezomib lacked viral replication factories within the cytoplasm. This was accompanied by the absence of late gene expression and DNA replication; however, early gene expression occurred unabated. Proteasomal inhibition with MG132 or bortezomib also had dramatic effects on viral titers, severely blocking viral replication and propagation. The effects of MG132 on poxvirus infection were reversible upon washout, resulting in the production of late genes and viral replication factories. Significantly, the addition of an ubiquitin-activating enzyme (E1) inhibitor had a similar affect on late and early protein expression. Together, our data suggests that a functional ubiquitin-proteasome system is required during poxvirus infection.
Figures


Similar articles
-
The ubiquitin-proteasome system plays an important role during various stages of the coronavirus infection cycle.J Virol. 2010 Aug;84(15):7869-79. doi: 10.1128/JVI.00485-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484504 Free PMC article.
-
Severe acute respiratory syndrome coronavirus replication is severely impaired by MG132 due to proteasome-independent inhibition of M-calpain.J Virol. 2012 Sep;86(18):10112-22. doi: 10.1128/JVI.01001-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787216 Free PMC article.
-
MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway.Sci Rep. 2020 Apr 21;10(1):6671. doi: 10.1038/s41598-020-63438-1. Sci Rep. 2020. PMID: 32317666 Free PMC article.
-
Targeting the proteasome pathway.Expert Opin Ther Targets. 2009 May;13(5):605-21. doi: 10.1517/14728220902866851. Expert Opin Ther Targets. 2009. PMID: 19397479 Review.
-
Manipulation of the ubiquitin-proteasome pathway by small DNA tumor viruses.Virology. 2009 Feb 20;384(2):317-23. doi: 10.1016/j.virol.2008.10.005. Epub 2008 Nov 14. Virology. 2009. PMID: 19013629 Review.
Cited by
-
Orthopoxvirus genes that mediate disease virulence and host tropism.Adv Virol. 2012;2012:524743. doi: 10.1155/2012/524743. Epub 2012 Jul 30. Adv Virol. 2012. PMID: 22899927 Free PMC article.
-
A vaccinia virus deletion mutant reveals the presence of additional inhibitors of NF-kappaB.J Virol. 2011 Jan;85(2):883-94. doi: 10.1128/JVI.01267-10. Epub 2010 Oct 27. J Virol. 2011. PMID: 20980497 Free PMC article.
-
The orthopoxvirus 68-kilodalton ankyrin-like protein is essential for DNA replication and complete gene expression of modified vaccinia virus Ankara in nonpermissive human and murine cells.J Virol. 2009 Jun;83(12):6029-38. doi: 10.1128/JVI.01628-08. Epub 2009 Apr 8. J Virol. 2009. PMID: 19357172 Free PMC article.
-
Selective modulation of cell surface proteins during vaccinia infection: A resource for identifying viral immune evasion strategies.PLoS Pathog. 2022 Jun 21;18(6):e1010612. doi: 10.1371/journal.ppat.1010612. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35727847 Free PMC article.
-
Virologs, viral mimicry, and virocell metabolism: the expanding scale of cellular functions encoded in the complex genomes of giant viruses.FEMS Microbiol Rev. 2023 Sep 5;47(5):fuad053. doi: 10.1093/femsre/fuad053. FEMS Microbiol Rev. 2023. PMID: 37740576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

