Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer
- PMID: 19099586
- PMCID: PMC2631013
- DOI: 10.1186/1471-2407-8-381
Class I histone deacetylases 1, 2 and 3 are highly expressed in renal cell cancer
Abstract
Background: Enhanced activity of histone deacetylases (HDAC) is associated with more aggressive tumour behaviour and tumour progression in various solid tumours. The over-expression of these proteins and their known functions in malignant neoplasms has led to the development of HDAC inhibitors (HDI) as new anti-neoplastic drugs. However, little is known about HDAC expression in renal cell cancer.
Methods: We investigated the expression of HDAC 1, 2 and 3 in 106 renal cell carcinomas and corresponding normal renal tissue by immunohistochemistry on tissue micro arrays and correlated expression data with clinico-pathological parameters including patient survival.
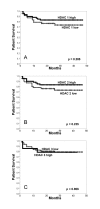
Results: Almost 60% of renal cell carcinomas expressed the HDAC isoforms 1 and 2. In contrast, HDAC 3 was only detected in 13% of all renal tumours, with particular low expression rates in the clear cell subtype. HDAC 3 was significantly higher expressed in pT1/2 tumours in comparison to pT3/4 tumours. Expression of class I HDAC isoforms correlated with each other and with the proliferative activity of the tumours. We found no prognostic value of the expression of any of the HDAC isoforms in this tumour entity.
Conclusion: Class I HDAC isoforms 1 and 2 are highly expressed in renal cell cancer, while HDAC 3 shows low, histology dependent expression rates. These unexpected differences in the expression patterns suggests alternative regulatory mechanisms of class I HDACs in renal cell cancer and should be taken into account when trials with isoform selective HDI are being planned. Whether HDAC expression in renal cancers is predictive of responsiveness for HDI will have to be tested in further studies.
Figures



Similar articles
-
Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy.Br J Cancer. 2008 Feb 12;98(3):604-10. doi: 10.1038/sj.bjc.6604199. Epub 2008 Jan 22. Br J Cancer. 2008. PMID: 18212746 Free PMC article.
-
Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis.Lancet Oncol. 2008 Feb;9(2):139-48. doi: 10.1016/S1470-2045(08)70004-4. Lancet Oncol. 2008. PMID: 18207460
-
HDAC 1 and 6 modulate cell invasion and migration in clear cell renal cell carcinoma.BMC Cancer. 2016 Aug 9;16:617. doi: 10.1186/s12885-016-2604-7. BMC Cancer. 2016. PMID: 27506904 Free PMC article.
-
HDAC expression and clinical prognosis in human malignancies.Cancer Lett. 2009 Aug 8;280(2):168-76. doi: 10.1016/j.canlet.2008.10.047. Epub 2008 Dec 21. Cancer Lett. 2009. PMID: 19103471 Review.
-
Explorative study on isoform-selective histone deacetylase inhibitors.Chem Pharm Bull (Tokyo). 2009 Sep;57(9):897-906. doi: 10.1248/cpb.57.897. Chem Pharm Bull (Tokyo). 2009. PMID: 19721249 Review.
Cited by
-
Tambulin Targets Histone Deacetylase 1 Inhibiting Cell Growth and Inducing Apoptosis in Human Lung Squamous Cell Carcinoma.Front Pharmacol. 2020 Aug 12;11:1188. doi: 10.3389/fphar.2020.01188. eCollection 2020. Front Pharmacol. 2020. PMID: 32903420 Free PMC article.
-
Clinical Significance of Histone Deacetylase (HDAC)-1, -2, -4 and -6 Expression in Salivary Gland Tumors.Diagnostics (Basel). 2021 Mar 14;11(3):517. doi: 10.3390/diagnostics11030517. Diagnostics (Basel). 2021. PMID: 33799478 Free PMC article.
-
Histone modifications: implications in renal cell carcinoma.Epigenomics. 2013 Aug;5(4):453-62. doi: 10.2217/epi.13.40. Epigenomics. 2013. PMID: 23895657 Free PMC article.
-
Histone deacetylase‑2: A potential regulator and therapeutic target in liver disease (Review).Int J Mol Med. 2021 Jul;48(1):131. doi: 10.3892/ijmm.2021.4964. Epub 2021 May 20. Int J Mol Med. 2021. PMID: 34013366 Free PMC article. Review.
-
Histone deacetylases (HDACs) in XPC gene silencing and bladder cancer.J Hematol Oncol. 2011 Apr 20;4:17. doi: 10.1186/1756-8722-4-17. J Hematol Oncol. 2011. PMID: 21507255 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

