Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer
- PMID: 19098006
- PMCID: PMC2640961
- DOI: 10.1074/jbc.M808591200
Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer
Abstract
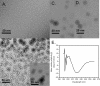
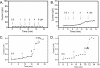
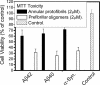
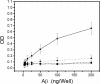
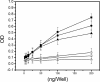
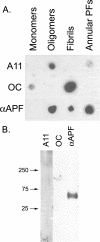
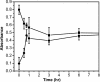
Amyloid oligomers are believed to play causal roles in several types of amyloid-related neurodegenerative diseases. Several different types of amyloid oligomers have been reported that differ in morphology, size, or toxicity, raising the question of the pathological significance and structural relationships between different amyloid oligomers. Annular protofibrils (APFs) have been described in oligomer preparations of many different amyloidogenic proteins and peptides as ring-shaped or pore-like structures. They are interesting because their pore-like morphology is consistent with numerous reports of membrane-permeabilizing activity of amyloid oligomers. Here we report the preparation of relatively homogeneous preparations of APFs and an antiserum selective for APFs (alphaAPF) compared with prefibrillar oligomers (PFOs) and fibrils. PFOs appear to be precursors for APF formation, which form in high yield after exposure to a hydrophobic-hydrophilic interface. Surprisingly, preformed APFs do not permeabilize lipid bilayers, unlike the precursor PFOs. APFs display a conformation-dependent, generic epitope that is distinct from that of PFOs and amyloid fibrils. Incubation of PFOs with phospholipids vesicles results in a loss of PFO immunoreactivity with a corresponding increase in alphaAPF immunoreactivity, suggesting that lipid vesicles catalyze the conversion of PFOs into APFs. The annular anti-protofibril antibody also recognizes heptameric alpha-hemolysin pores, but not monomers, suggesting that the antibody recognizes an epitope that is specific for a beta barrel structural motif.
Figures


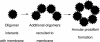
Similar articles
-
Fibrillar oligomers nucleate the oligomerization of monomeric amyloid beta but do not seed fibril formation.J Biol Chem. 2010 Feb 26;285(9):6071-9. doi: 10.1074/jbc.M109.069542. Epub 2009 Dec 15. J Biol Chem. 2010. PMID: 20018889 Free PMC article.
-
Structural classification of toxic amyloid oligomers.J Biol Chem. 2008 Oct 31;283(44):29639-43. doi: 10.1074/jbc.R800016200. Epub 2008 Aug 22. J Biol Chem. 2008. PMID: 18723507 Free PMC article. Review.
-
Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases.J Biol Chem. 2004 Nov 5;279(45):46363-6. doi: 10.1074/jbc.C400260200. Epub 2004 Sep 21. J Biol Chem. 2004. PMID: 15385542
-
Astrocytes contain amyloid-β annular protofibrils in Alzheimer's disease brains.FEBS Lett. 2011 Oct 3;585(19):3052-7. doi: 10.1016/j.febslet.2011.08.027. Epub 2011 Aug 24. FEBS Lett. 2011. PMID: 21872592
-
New Mechanism of Amyloid Fibril Formation.Curr Protein Pept Sci. 2019;20(6):630-640. doi: 10.2174/1389203720666190125160937. Curr Protein Pept Sci. 2019. PMID: 30686252 Review.
Cited by
-
Morphological and Molecular Profiling of Amyloid-β Species in Alzheimer's Pathogenesis.Mol Neurobiol. 2024 Oct 24. doi: 10.1007/s12035-024-04543-4. Online ahead of print. Mol Neurobiol. 2024. PMID: 39446217 Review.
-
Alzheimers disease: review of emerging treatment role for intravenous immunoglobulins.J Cent Nerv Syst Dis. 2011 May 8;3:67-73. doi: 10.4137/JCNSD.S5018. Print 2011. J Cent Nerv Syst Dis. 2011. PMID: 23861639 Free PMC article.
-
Function and dysfunction of α-synuclein: probing conformational changes and aggregation by single molecule fluorescence.Mol Neurobiol. 2013 Apr;47(2):622-31. doi: 10.1007/s12035-012-8338-x. Epub 2012 Sep 16. Mol Neurobiol. 2013. PMID: 22983916 Free PMC article. Review.
-
Isolation and characterization of antibody fragments selective for toxic oligomeric tau.Neurobiol Aging. 2015 Mar;36(3):1342-55. doi: 10.1016/j.neurobiolaging.2014.12.002. Epub 2014 Dec 11. Neurobiol Aging. 2015. PMID: 25616912 Free PMC article.
-
Role of prion protein aggregation in neurotoxicity.Int J Mol Sci. 2012;13(7):8648-8669. doi: 10.3390/ijms13078648. Epub 2012 Jul 11. Int J Mol Sci. 2012. PMID: 22942726 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

