Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene
- PMID: 19095962
- PMCID: PMC2631318
- DOI: 10.2353/ajpath.2009.080602
Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene
Abstract
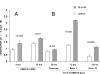
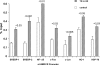
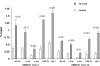
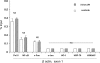
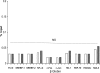
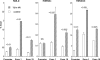
Acute kidney injury evokes renal tubular cholesterol synthesis. However, the factors during acute kidney injury that regulate HMG CoA reductase (HMGCR) activity, the rate-limiting step in cholesterol synthesis, have not been defined. To investigate these factors, mice were subjected to 30 minutes of either unilateral renal ischemia or sham surgery. After 3 days, bilateral nephrectomy was performed and cortical tissue extracts were prepared. The recruitment of RNA polymerase II (Pol II), transcription factors (SREBP-1, SREBP-2, NF-kappaB, c-Fos, and c-Jun), and heat shock proteins (HSP-70 and heme oxygenase-1) to the HMGCR promoter and transcription region (start/end exons) were assessed by Matrix ChIP assay. HMGCR mRNA, protein, and cholesterol levels were determined. Finally, histone modifications at HMGCR were assessed. Ischemia/reperfusion (I/R) induced marked cholesterol loading, which corresponded with elevated Pol II recruitment to HMGCR and increased expression levels of both HMGCR protein and mRNA. I/R also induced the binding of multiple transcription factors (SREBP-1, SREBP-2, c-Fos, c-Jun, NF-kappaB) and heat shock proteins to the HMGCR promoter and transcription regions. Significant histone modifications (increased H3K4m3, H3K19Ac, and H2A.Z variant) at these loci were also observed but were not identified at either the 5' and 3' HMGCR flanking regions (+/-5000 bps) or at negative control genes (beta-actin and beta-globin). In conclusion, I/R activates the HMGCR gene via multiple stress-activated transcriptional and epigenetic pathways, contributing to renal cholesterol loading.
Figures


Similar articles
-
HMG-CoA reductase activation and urinary pellet cholesterol elevations in acute kidney injury.Clin J Am Soc Nephrol. 2011 Sep;6(9):2108-13. doi: 10.2215/CJN.02440311. Epub 2011 Jul 28. Clin J Am Soc Nephrol. 2011. PMID: 21799150 Free PMC article.
-
New insights into cellular cholesterol acquisition: promoter analysis of human HMGCR and SQLE, two key control enzymes in cholesterol synthesis.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jul;1862(7):647-657. doi: 10.1016/j.bbalip.2017.03.009. Epub 2017 Mar 23. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28342963
-
The mevalonate pathway during acute tubular injury: selected determinants and consequences.Am J Pathol. 2002 Aug;161(2):681-92. doi: 10.1016/S0002-9440(10)64224-1. Am J Pathol. 2002. PMID: 12163393 Free PMC article.
-
Feedback and hormonal regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: the concept of cholesterol buffering capacity.Proc Soc Exp Biol Med. 2000 May;224(1):8-19. doi: 10.1046/j.1525-1373.2000.22359.x. Proc Soc Exp Biol Med. 2000. PMID: 10782041 Review.
-
A second gene for peroxisomal HMG-CoA reductase? A genomic reassessment.J Lipid Res. 2002 Dec;43(12):2031-6. doi: 10.1194/jlr.r200010-jlr200. J Lipid Res. 2002. PMID: 12454262 Review.
Cited by
-
HMG-CoA reductase activation and urinary pellet cholesterol elevations in acute kidney injury.Clin J Am Soc Nephrol. 2011 Sep;6(9):2108-13. doi: 10.2215/CJN.02440311. Epub 2011 Jul 28. Clin J Am Soc Nephrol. 2011. PMID: 21799150 Free PMC article.
-
Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and "end-stage" kidney disease.Am J Physiol Renal Physiol. 2011 Dec;301(6):F1334-45. doi: 10.1152/ajprenal.00431.2011. Epub 2011 Sep 14. Am J Physiol Renal Physiol. 2011. PMID: 21921025 Free PMC article.
-
Early lipid changes in acute kidney injury using SWATH lipidomics coupled with MALDI tissue imaging.Am J Physiol Renal Physiol. 2016 May 15;310(10):F1136-47. doi: 10.1152/ajprenal.00100.2016. Epub 2016 Feb 24. Am J Physiol Renal Physiol. 2016. PMID: 26911846 Free PMC article.
-
Dysregulation of histone H3 lysine 27 trimethylation in transforming growth factor-β1-induced gene expression in mesangial cells and diabetic kidney.J Biol Chem. 2019 Aug 23;294(34):12695-12707. doi: 10.1074/jbc.RA119.007575. Epub 2019 Jul 2. J Biol Chem. 2019. PMID: 31266808 Free PMC article.
-
The Contribution of Histone Crotonylation to Tissue Health and Disease: Focus on Kidney Health.Front Pharmacol. 2020 Apr 3;11:393. doi: 10.3389/fphar.2020.00393. eCollection 2020. Front Pharmacol. 2020. PMID: 32308622 Free PMC article. Review.
References
-
- Honda N, Hishida A, Ikuma K, Yonemura K. Acquired resistance to acute renal failure. Kidney Int. 1987;31:1233–1238. - PubMed
-
- Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. - PubMed
-
- Reimer KA, Murry CE, Jennings RB. Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation. 1990;82:2266–2268. - PubMed
-
- Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2:313–318. - PubMed
-
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

