The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics
- PMID: 19090622
- PMCID: PMC2602726
- DOI: 10.1371/journal.pbio.0060319
The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics
Expression of concern in
-
Expression of Concern: The Communication Factor EDF and the Toxin-Antitoxin Module mazEF Determine the Mode of Action of Antibiotics.PLoS Biol. 2021 May 10;19(5):e3001246. doi: 10.1371/journal.pbio.3001246. eCollection 2021 May. PLoS Biol. 2021. PMID: 33970898 Free PMC article. No abstract available.
Abstract
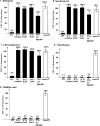
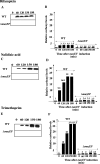
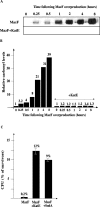
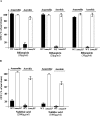
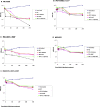
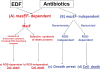
It was recently reported that the production of Reactive Oxygen Species (ROS) is a common mechanism of cell death induced by bactericidal antibiotics. Here we show that triggering the Escherichia coli chromosomal toxin-antitoxin system mazEF is an additional determinant in the mode of action of some antibiotics. We treated E. coli cultures by antibiotics belonging to one of two groups: (i) Inhibitors of transcription and/or translation, and (ii) DNA damaging. We found that antibiotics of both groups caused: (i) mazEF-mediated cell death, and (ii) the production of ROS through MazF action. However, only antibiotics of the first group caused mazEF-mediated cell death that is ROS-dependent, whereas those of the second group caused mazEF-mediated cell death by an ROS-independent pathway. Furthermore, our results showed that the mode of action of antibiotics was determined by the ability of E. coli cells to communicate through the signaling molecule Extracellular Death Factor (EDF) participating in mazEF induction.
Conflict of interest statement
Figures

Comment in
-
Editorial Note: The Communication Factor EDF and the Toxin-Antitoxin Module mazEF Determine the Mode of Action of Antibiotics.PLoS Biol. 2023 Sep 18;21(9):e3002325. doi: 10.1371/journal.pbio.3002325. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37721929 Free PMC article. No abstract available.
Similar articles
-
A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli.Science. 2007 Oct 26;318(5850):652-5. doi: 10.1126/science.1147248. Science. 2007. PMID: 17962566
-
Two programmed cell death systems in Escherichia coli: an apoptotic-like death is inhibited by the mazEF-mediated death pathway.PLoS Biol. 2012;10(3):e1001281. doi: 10.1371/journal.pbio.1001281. Epub 2012 Mar 6. PLoS Biol. 2012. PMID: 22412352 Free PMC article.
-
Programmed cell death in Escherichia coli: some antibiotics can trigger mazEF lethality.J Bacteriol. 2001 Mar;183(6):2041-5. doi: 10.1128/JB.183.6.2041-2045.2001. J Bacteriol. 2001. PMID: 11222603 Free PMC article.
-
Quorum sensing peptides mediating interspecies bacterial cell death as a novel class of antimicrobial agents.Curr Opin Microbiol. 2014 Oct;21:22-7. doi: 10.1016/j.mib.2014.09.001. Epub 2014 Sep 20. Curr Opin Microbiol. 2014. PMID: 25244032 Review.
-
mazEF-mediated programmed cell death in bacteria: "what is this?".Crit Rev Microbiol. 2015 Feb;41(1):89-100. doi: 10.3109/1040841X.2013.804030. Epub 2013 Jun 25. Crit Rev Microbiol. 2015. PMID: 23799870 Review.
Cited by
-
How antibiotics kill bacteria: from targets to networks.Nat Rev Microbiol. 2010 Jun;8(6):423-35. doi: 10.1038/nrmicro2333. Epub 2010 May 4. Nat Rev Microbiol. 2010. PMID: 20440275 Free PMC article. Review.
-
Activation of a built-in bacterial programmed cell death system as a novel mechanism of action of some antibiotics.Commun Integr Biol. 2009 May;2(3):211-2. doi: 10.4161/cib.2.3.7876. Commun Integr Biol. 2009. PMID: 19641731 Free PMC article.
-
Ribosomal elongation factor 4 promotes cell death associated with lethal stress.mBio. 2014 Dec 9;5(6):e01708. doi: 10.1128/mBio.01708-14. mBio. 2014. PMID: 25491353 Free PMC article.
-
Combined Systems Approaches Reveal a Multistage Mode of Action of a Marine Antimicrobial Peptide against Pathogenic Escherichia coli and Its Protective Effect against Bacterial Peritonitis and Endotoxemia.Antimicrob Agents Chemother. 2016 Dec 27;61(1):e01056-16. doi: 10.1128/AAC.01056-16. Print 2017 Jan. Antimicrob Agents Chemother. 2016. PMID: 27795369 Free PMC article.
-
Potential roles for DNA replication and repair functions in cell killing by streptomycin.Mutat Res. 2013 Sep;749(1-2):87-91. doi: 10.1016/j.mrfmmm.2013.07.009. Epub 2013 Aug 17. Mutat Res. 2013. PMID: 23958411 Free PMC article.
References
-
- Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infection. Clin Infect Dis. 2004;38:864–870. - PubMed
-
- Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. - PubMed
-
- Kohanski MA, Dwyer DJ, Hayate B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bacterial antibiotics. Cell. 2007;130:797–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

