Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics
- PMID: 19088204
- PMCID: PMC2603432
- DOI: 10.1073/pnas.0811411106
Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics
Abstract
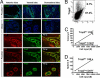
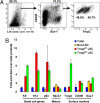
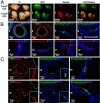
The epithelium of the adult prostate contains 3 distinct cell types: basal, luminal, and neuroendocrine. Tissue-regenerative activity has been identified predominantly from the basal cells, isolated by expression of CD49f and stem cell antigen-1 (Sca-1). An important question for the field is whether all basal cells have stem cell characteristics. Prostate-specific microarray databases were interrogated to find candidate surface antigens that could subfractionate the basal cell population. Tumor-associated calcium signal transducer 2 (TACSTD2/Trop2/M1S1/GA733-1) was identified because it was enriched after castration, in prostate sphere cells and in the basal fraction. In the murine prostate, Trop2 shows progenitor characteristics such as localization to the region of the gland proximal to the urethra and enrichment for sphere-forming and colony-forming cells. Trop2 subfractionates the basal cells into 2 populations, both of which express characteristic basal cell markers by quantitative PCR. However, only the basal cells expressing high levels of Trop2 were able to efficiently form spheres in vitro. In the human prostate, where Sca-1 is not expressed, sphere-forming progenitor cells were also isolated based on high expression of Trop2 and CD49f. Trop2-expressing murine basal cells could regenerate prostatic tubules in vivo, whereas the remaining basal cells had minimal activity. Evidence was found for basal, luminal, and neuroendocrine cells in prostatic tubules regenerated from Trop2(hi) basal cells. In summary, functionally distinct populations of cells exist within the prostate basal compartment and an epithelial progenitor can give rise to neuroendocrine cells in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Protein profile of basal prostate epithelial progenitor cells--stage-specific embryonal antigen 4 expressing cells have enhanced regenerative potential in vivo.J Cell Mol Med. 2016 Apr;20(4):721-30. doi: 10.1111/jcmm.12785. Epub 2016 Feb 5. J Cell Mol Med. 2016. PMID: 26849468 Free PMC article.
-
The influence of prostatic anatomy and neurotrophins on basal prostate epithelial progenitor cells.Prostate. 2016 Jan;76(1):114-21. doi: 10.1002/pros.23109. Epub 2015 Oct 7. Prostate. 2016. PMID: 26444457
-
Epcam, CD44, and CD49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability.PLoS One. 2012;7(4):e34219. doi: 10.1371/journal.pone.0034219. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514625 Free PMC article.
-
Epithelial stem cells of the prostate and their role in cancer progression.Cold Spring Harb Symp Quant Biol. 2008;73:491-502. doi: 10.1101/sqb.2008.73.012. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022743 Review.
-
Prostate stem cells: the niche and cell markers.Int J Urol. 2008 Apr;15(4):289-94. doi: 10.1111/j.1442-2042.2008.02047.x. Int J Urol. 2008. PMID: 18380813 Review.
Cited by
-
Differential gene expression profiling of functionally and developmentally distinct human prostate epithelial populations.Prostate. 2015 May;75(7):764-76. doi: 10.1002/pros.22959. Epub 2015 Feb 7. Prostate. 2015. PMID: 25663004 Free PMC article.
-
Notch and TGFβ form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity.Cell Stem Cell. 2012 Nov 2;11(5):676-88. doi: 10.1016/j.stem.2012.07.003. Cell Stem Cell. 2012. PMID: 23122291 Free PMC article.
-
A plethora of progenitors in the post-natal prostate.EMBO Rep. 2012 Dec;13(12):1036-7. doi: 10.1038/embor.2012.169. Epub 2012 Nov 9. EMBO Rep. 2012. PMID: 23138032 Free PMC article. No abstract available.
-
Activation of silenced tumor suppressor genes in prostate cancer cells by a novel energy restriction-mimetic agent.Prostate. 2012 Dec 1;72(16):1767-78. doi: 10.1002/pros.22530. Epub 2012 Apr 26. Prostate. 2012. PMID: 22539223 Free PMC article.
-
Trop2 Forms a Stable Dimer with Significant Structural Differences within the Membrane-Distal Region as Compared to EpCAM.Int J Mol Sci. 2021 Sep 30;22(19):10640. doi: 10.3390/ijms221910640. Int J Mol Sci. 2021. PMID: 34638982 Free PMC article.
References
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Passegue E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. - PubMed
-
- English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. - PubMed
-
- Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

