Tocopheryl Polyethylene Glycol Succinate as a Safe, Antioxidant Surfactant for Processing Carbon Nanotubes and Fullerenes
- PMID: 19081834
- PMCID: PMC2598771
- DOI: 10.1016/j.carbon.2007.08.035
Tocopheryl Polyethylene Glycol Succinate as a Safe, Antioxidant Surfactant for Processing Carbon Nanotubes and Fullerenes
Abstract
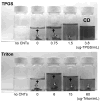
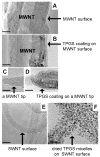
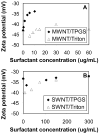
This work investigates the physical interactions between carbon nanomaterials and tocopheryl polyethylene glycol succinate (TPGS). TPGS is a synthetic amphiphile that undergoes enzymatic cleavage to deliver the lipophilic antioxidant, alpha-tocopherol (vitamin E) to cell membranes, and is FDA approved as a water-soluble vitamin E nutritional supplement and drug delivery vehicle. Here we show that TPGS 1000 is capable of dispersing multi-wall and single-wall carbon nanotubes in aqueous media, and for multiwall tubes is more effective than the commonly used non-ionic surfactant Triton X-100. TPGS is also capable of solubilizing C(60) in aqueous phases by dissolving fullerene in the core of its spherical micelles. Drying of these solutions leads to fullerene/TPGS phase separation and the self-assembly of highly ordered asymmetric nanoparticles, with fullerene nanocrystals attached to the hydrophobic end of crystalline TPGS nanobrushes. The article discusses surface charge, colloidal stability, and the potential applications of TPGS as a safe surfactant for "green" processing of carbon nanomaterials.
Figures



Similar articles
-
The applications of Vitamin E TPGS in drug delivery.Eur J Pharm Sci. 2013 May 13;49(2):175-86. doi: 10.1016/j.ejps.2013.02.006. Epub 2013 Feb 26. Eur J Pharm Sci. 2013. PMID: 23485439 Review.
-
Morphology, gelation and cytotoxicity evaluation of D-α-Tocopheryl polyethylene glycol succinate (TPGS) - Tetronic mixed micelles.J Colloid Interface Sci. 2021 Jan 15;582(Pt A):353-363. doi: 10.1016/j.jcis.2020.08.004. Epub 2020 Aug 3. J Colloid Interface Sci. 2021. PMID: 32858401
-
Investigation of the micellar properties of the tocopheryl polyethylene glycol succinate surfactants TPGS 400 and TPGS 1000 by steady state fluorometry.J Colloid Interface Sci. 2009 May 15;333(2):585-9. doi: 10.1016/j.jcis.2009.01.048. Epub 2009 Feb 20. J Colloid Interface Sci. 2009. PMID: 19232633
-
Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery.Mol Pharm. 2018 Feb 5;15(2):571-584. doi: 10.1021/acs.molpharmaceut.7b00939. Epub 2018 Jan 24. Mol Pharm. 2018. PMID: 29313693
-
TPGS Decorated Liposomes as Multifunctional Nano-Delivery Systems.Pharm Res. 2023 Jan;40(1):245-263. doi: 10.1007/s11095-022-03424-6. Epub 2022 Nov 14. Pharm Res. 2023. PMID: 36376604 Free PMC article. Review.
Cited by
-
Photodynamic therapy with fullerenes in vivo: reality or a dream?Nanomedicine (Lond). 2011 Dec;6(10):1813-25. doi: 10.2217/nnm.11.144. Nanomedicine (Lond). 2011. PMID: 22122587 Free PMC article.
-
Iron Transport Tocopheryl Polyethylene Glycol Succinate in Animal Health and Diseases.Molecules. 2019 Nov 25;24(23):4289. doi: 10.3390/molecules24234289. Molecules. 2019. PMID: 31775281 Free PMC article. Review.
-
Can nanotechnology potentiate photodynamic therapy?Nanotechnol Rev. 2012 Mar;1(2):111-146. doi: 10.1515/ntrev-2011-0005. Nanotechnol Rev. 2012. PMID: 26361572 Free PMC article.
-
Antioxidant deactivation on graphenic nanocarbon surfaces.Small. 2011 Oct 4;7(19):2775-85. doi: 10.1002/smll.201100651. Epub 2011 Aug 5. Small. 2011. PMID: 21818846 Free PMC article.
-
Development and mechanistic study of a microemulsion containing vitamin E TPGS for the enhancement of oral absorption of celecoxib.Int J Nanomedicine. 2019 Apr 30;14:3087-3102. doi: 10.2147/IJN.S201449. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31118624 Free PMC article.
References
-
- Vaisman L, Wagner HD, Marom G. The role of surfactants in dispersion of carbon nanotubes. Adv Colloid Interface Sci. 2006;128–130:37–46. - PubMed
-
- Balavoine F, Schultz P, Richard C, Mallouh V, Ebbesen TW, Mioskowski C. Helical crystallization of proteins on carbon nanotubes: a first step towards the development of new biosensors. Angew Chem Int Ed. 1999;38(13–14):1912–5. - PubMed
-
- Karajanagi SS, Yang HC, Asuri P, Sellitto E, Dordick JS, Kane RS. Protein-assisted solubilization of single-walled carbon nanotubes. Langmuir. 2006;22(4):1392–5. - PubMed
-
- O’Connell MJ, Boul P, Ericson LM, Huffman C, Wang Y, Haroz E, et al. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem Phys Lett. 2001;342:265–71.
-
- Islam MF, Rojas E, Bergey DM, Johnson AT, Yodh AG. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003;3(2):269–73.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
