Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice
- PMID: 19079608
- PMCID: PMC2597179
- DOI: 10.1371/journal.pone.0003950
Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice
Abstract
Background: Macrophages, key regulators of healing/regeneration processes, strongly infiltrate ischemic tissues from patients suffering from critical limb ischemia (CLI). However pro-inflammatory markers correlate with disease progression and risk of amputation, suggesting that modulating macrophage activation state might be beneficial. We previously reported that thrombospondin-1 (TSP-1) is highly expressed in ischemic tissues during CLI in humans. TSP-1 is a matricellular protein that displays well-known angiostatic properties in cancer, and regulates inflammation in vivo and macrophages properties in vitro. We therefore sought to investigate its function in a mouse model of CLI.
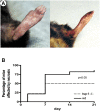
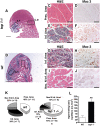
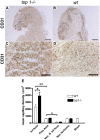
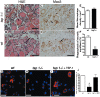
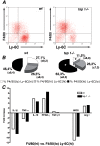
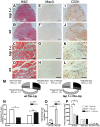
Methods and findings: Using a genetic model of tsp-1(-/-) mice subjected to femoral artery excision, we report that tsp-1(-/-) mice were clinically and histologically protected from necrosis compared to controls. Tissue protection was associated with increased postischemic angiogenesis and muscle regeneration. We next showed that macrophages present in ischemic tissues exhibited distinct phenotypes in tsp-1(-/-) and wt mice. A strong reduction of necrotic myofibers phagocytosis was observed in tsp-1(-/-) mice. We next demonstrated that phagocytosis of muscle cell debris is a potent pro-inflammatory signal for macrophages in vitro. Consistently with these findings, macrophages that infiltrated ischemic tissues exhibited a reduced postischemic pro-inflammatory activation state in tsp-1(-/-) mice, characterized by a reduced Ly-6C expression and a less pro-inflammatory cytokine expression profile. Finally, we showed that monocyte depletion reversed clinical and histological protection from necrosis observed in tsp-1(-/-) mice, thereby demonstrating that macrophages mediated tissue protection in these mice.
Conclusion: This study defines targeting postischemic macrophage activation state as a new potential therapeutic approach to protect tissues from necrosis and promote tissue repair during CLI. Furthermore, our data suggest that phagocytosis plays a crucial role in promoting a deleterious intra-tissular pro-inflammatory macrophage activation state during critical injuries. Finally, our results describe TSP-1 as a new relevant physiological target during critical leg ischemia.
Conflict of interest statement
Figures

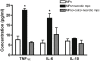
Similar articles
-
Thrombospondin-1 and disease progression in dysferlinopathy.Hum Mol Genet. 2017 Dec 15;26(24):4951-4960. doi: 10.1093/hmg/ddx378. Hum Mol Genet. 2017. PMID: 29206970
-
Thrombospondin-1 levels correlate with macrophage activity and disease progression in dysferlin deficient mice.Neuromuscul Disord. 2016 Mar;26(3):240-51. doi: 10.1016/j.nmd.2016.01.002. Epub 2016 Jan 26. Neuromuscul Disord. 2016. PMID: 26927626
-
Effects of thrombospondin-4 on pro-inflammatory phenotype differentiation and apoptosis in macrophages.Cell Death Dis. 2020 Jan 23;11(1):53. doi: 10.1038/s41419-020-2237-2. Cell Death Dis. 2020. PMID: 31974349 Free PMC article.
-
Human expression patterns: qualitative and quantitative analysis of thrombospondin-1 under physiological and pathological conditions.J Cell Mol Med. 2018 Apr;22(4):2086-2097. doi: 10.1111/jcmm.13565. Epub 2018 Feb 14. J Cell Mol Med. 2018. PMID: 29441713 Free PMC article. Review.
-
Protective and pathogenic functions of macrophage subsets.Nat Rev Immunol. 2011 Oct 14;11(11):723-37. doi: 10.1038/nri3073. Nat Rev Immunol. 2011. PMID: 21997792 Free PMC article. Review.
Cited by
-
Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms.Stem Cells. 2013 Jan;31(1):117-25. doi: 10.1002/stem.1263. Stem Cells. 2013. PMID: 23097349 Free PMC article.
-
Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model.Korean J Physiol Pharmacol. 2016 Nov;20(6):657-667. doi: 10.4196/kjpp.2016.20.6.657. Epub 2016 Oct 28. Korean J Physiol Pharmacol. 2016. PMID: 27847443 Free PMC article.
-
Macrophage-mediated PDGF Activation Correlates With Regenerative Outcomes Following Musculoskeletal Trauma.Ann Surg. 2023 Aug 1;278(2):e349-e359. doi: 10.1097/SLA.0000000000005704. Epub 2022 Sep 15. Ann Surg. 2023. PMID: 36111847 Free PMC article.
-
Functions of Thrombospondin-1 in the Tumor Microenvironment.Int J Mol Sci. 2021 Apr 27;22(9):4570. doi: 10.3390/ijms22094570. Int J Mol Sci. 2021. PMID: 33925464 Free PMC article. Review.
-
N-acetylcysteine differentially regulates the populations of bone marrow and circulating endothelial progenitor cells in mice with limb ischemia.Eur J Pharmacol. 2020 Aug 15;881:173233. doi: 10.1016/j.ejphar.2020.173233. Epub 2020 May 31. Eur J Pharmacol. 2020. PMID: 32492379 Free PMC article.
References
-
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31:S1–S296. - PubMed
-
- Barani J, Nilsson JA, Mattiasson I, Lindblad B, Gottsater A. Inflammatory mediators are associated with 1-year mortality in critical limb ischemia. J Vasc Surg. 2005;42:75–80. - PubMed
-
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. - PubMed
-
- Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. - PubMed
-
- Rajagopalan S, Mohler E, 3rd, Lederman RJ, Saucedo J, Mendelsohn FO, et al. Regional Angiogenesis with Vascular Endothelial Growth Factor (VEGF) in peripheral arterial disease: Design of the RAVE trial. Am Heart J. 2003;145:1114–1118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

