Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection
- PMID: 19075290
- PMCID: PMC2605225
- DOI: 10.1084/jem.20081661
Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection
Abstract
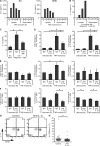
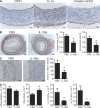
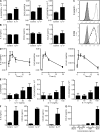
Interleukin (IL) 1alpha produced by human endothelial cells (ECs), in response to tumor necrosis factor (TNF) or to co-culture with allogeneic T cells in a TNF-dependent manner, can augment the release of cytokines from alloreactive memory T cells in vitro. In a human-mouse chimeric model of artery allograft rejection, ECs lining the transplanted human arteries express IL-1alpha, and blocking IL-1 reduces the extent of human T cell infiltration into the artery intima and selectively inhibits IL-17 production by infiltrating T cells. In human skin grafts implanted on immunodeficient mice, administration of IL-17 is sufficient to induce mild inflammation. In cultured cells, IL-17 acts preferentially on vascular smooth muscle cells rather than ECs to enhance production of proinflammatory mediators, including IL-6, CXCL8, and CCL20. Neutralization of IL-17 does not reduce T cell infiltration into allogeneic human artery grafts, but markedly reduces IL-6, CXCL8, and CCL20 expression and selectively inhibits CCR6(+) T cell accumulation in rejecting arteries. We conclude that graft-derived IL-1 can promote T cell intimal recruitment and IL-17 production during human artery allograft rejection, and suggest that targeting IL-1 in the perioperative transplant period may modulate host alloreactivity.
Figures

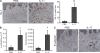
Similar articles
-
IL-1alpha and IL-1beta are endogenous mediators linking cell injury to the adaptive alloimmune response.J Immunol. 2007 Nov 15;179(10):6536-46. doi: 10.4049/jimmunol.179.10.6536. J Immunol. 2007. PMID: 17982042
-
Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells.J Clin Invest. 2013 Apr;123(4):1677-93. doi: 10.1172/JCI66204. Epub 2013 Mar 8. J Clin Invest. 2013. PMID: 23478407 Free PMC article.
-
Peroxisome proliferator-activated receptor-γ agonists prevent in vivo remodeling of human artery induced by alloreactive T cells.Circulation. 2011 Jul 12;124(2):196-205. doi: 10.1161/CIRCULATIONAHA.110.015396. Epub 2011 Jun 20. Circulation. 2011. PMID: 21690493 Free PMC article.
-
Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse.Springer Semin Immunopathol. 2003 Sep;25(2):167-80. doi: 10.1007/s00281-003-0135-1. Springer Semin Immunopathol. 2003. PMID: 12955465 Review.
-
Endothelial injury, alarmins, and allograft rejection.Crit Rev Immunol. 2008;28(3):229-48. doi: 10.1615/critrevimmunol.v28.i3.40. Crit Rev Immunol. 2008. PMID: 19024347 Review.
Cited by
-
Interleukin 17 in vascular inflammation.Cytokine Growth Factor Rev. 2010 Dec;21(6):463-9. doi: 10.1016/j.cytogfr.2010.10.003. Epub 2010 Nov 12. Cytokine Growth Factor Rev. 2010. PMID: 21075042 Free PMC article. Review.
-
B cell-derived IL-1β and IL-6 drive T cell reconstitution following lymphoablation.Am J Transplant. 2020 Oct;20(10):2740-2754. doi: 10.1111/ajt.15960. Epub 2020 May 16. Am J Transplant. 2020. PMID: 32342598 Free PMC article.
-
Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells.J Immunol. 2011 Dec 15;187(12):6268-80. doi: 10.4049/jimmunol.1003774. Epub 2011 Nov 14. J Immunol. 2011. PMID: 22084439 Free PMC article.
-
Experimental models of cardiac transplantation: design determines relevance.Curr Opin Organ Transplant. 2014 Oct;19(5):525-30. doi: 10.1097/MOT.0000000000000113. Curr Opin Organ Transplant. 2014. PMID: 25160697 Free PMC article. Review.
-
Association of Pericardiac Adipose Tissue With Coronary Artery Disease.Front Endocrinol (Lausanne). 2021 Sep 6;12:724859. doi: 10.3389/fendo.2021.724859. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34552562 Free PMC article.
References
-
- Libby, P., and J.S. Pober. 2001. Chronic rejection. Immunity. 14:387–397. - PubMed
-
- Nickeleit, V., E.C. Vamvakas, M. Pascual, B.J. Poletti, and R.B. Colvin. 1998. The prognostic significance of specific arterial lesions in acute renal allograft rejection. J. Am. Soc. Nephrol. 9:1301–1308. - PubMed
-
- Racusen, L.C., K. Solez, R.B. Colvin, S.M. Bonsib, M.C. Castro, T. Cavallo, B.P. Croker, A.J. Demetris, C.B. Drachenberg, A.B. Fogo, et al. 1999. The Banff 97 working classification of renal allograft pathology. Kidney Int. 55:713–723. - PubMed
-
- Trpkov, K., P. Campbell, F. Pazderka, S. Cockfield, K. Solez, and P.F. Halloran. 1996. Pathologic features of acute renal allograft rejection associated with donor-specific antibody: analysis using the Banff grading schema. Transplantation. 61:1586–1592. - PubMed
-
- Choi, J., D.R. Enis, K.P. Koh, S.L. Shiao, and J.S. Pober. 2004. T lymphocyte-endothelial cell interactions. Annu. Rev. Immunol. 22:683–709. - PubMed

